Abstract
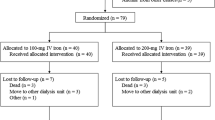
Intravenous iron therapy is recommended for children and adults who receive hemodialysis (HD) and recombinant human erythropoietin (rHuEPO). However, limited information exists on the use of any maintenance IV iron regimen in children. Therefore, we conducted a prospective, multicenter, open-label trial of maintenance therapy with sodium ferric gluconate complex (SFGC) in iron-replete pediatric HD patients receiving rHuEPO. Patients received SFGC weekly at an initial dose of 1.0 mg kg−1 week−1, not to exceed 125 mg. Doses could be adjusted based on iron indices. Twenty-three patients (mean age: 13.2±2.39 years) were enrolled and received at least one dose of SFGC, while twelve patients completed the study. After 12 weeks of treatment, the mean SFGC dose delivered was 1.0 mg/kg. Mean TSAT and serum ferritin levels remained within NKF-K/DOQI target ranges and the mean Hgb level remained unchanged from baseline. No unexpected or unusual safety risks were associated with SFGC use. In summary, this experience provides evidence for the safety and efficacy of intravenous SFGC and supports the recommendation that the maintenance SFGC starting dose should be 1.0 mg/kg, not to exceed 125 mg, with subsequent adjustments made according to TSAT and/or serum ferritin levels.


Similar content being viewed by others
References
National Kidney Foundation-K/DOQI (2001) National kidney foundation-K/DOQI clinical practice guidelines for anemia of chronic kidney disease. Am J Kidney Dis 37 (Suppl1):S182–S238
Fishbane S, Frei GL, Maesaka J (1995) Reduction in recombinant human erythropoietin doses by the use of chronic intravenous iron supplementation. Am J Kidney Dis 26:41–46
Silverberg DS, Blum M, Peer G, Kaplan F, Iaina A (1996) Intravenous ferric saccharate as an iron supplement in dialysis patients. Nephron 72:413–417
Macdougall IC, Tucker B, Thompson J, Tomson CRV, Baker LRI, Raine AEG (1996) A randomized controlled study of iron supplementation in patients treated with erythropoietin. Kidney Int 50:1694–1699
Granolleras C, Oules R, Branger B, Fourcade J, Shaldon S (1993) Iron supplementation of hemodialysis patients receiving recombinant human erythropoietin therapy. In: Bauer C, Koch KM, Scigalla P, Wieczorek L (eds) Erythropoietin: molecular physiology and clinical applications. Marcel Dekker, New York, pp 211–218
Taylor JE, Peat N, Porter C, Morgan AG (1996) Regular, low-dose intravenous iron therapy improves response to erythropoietin in haemodialysis patients. Nephrol Dial Transplant 11:1079–1083
Sepandj F, Jindal K, West M, Hirsch D (1996) Economic appraisal of maintenance parenteral iron administration in treatment of the anaemia in chronic haemodialysis patients. Nephrol Dial Transplant 11:319–322
Suh H, Wadhwa NK (1992) Iron dextran treatment in peritoneal dialysis patients on erythropoietin. Adv Perit Dial 8:464–466
Senger JM, Weiss RJ (1996) Hematologic and erythropoietin responses to iron dextran in the hemodialysis environment. ANNA J 23:319–323
Leonard MB, Donaldson LA, Ho M, Geary DF (2003) A prospective cohort study of incident maintenance dialysis in children: an NAPRTCS study. Kidney Int 63:744–755
Fishbane S, Ungureanu VD, Maeska JK, Kaupke CJ, Lim V, Wish J (1996) The safety of intravenous iron dextran in hemodialysis patients. Am J Kidney Dis 30:907–911
Michael B, Coyne DW, Fishbane S, Folkert V, Lynn RI, Nissenson AR, Agarwal R, Eschbach JW, Fadem SZ, Trout JR, Strobos J, Warnock DG; Ferrlecit Publication Committee (2002) Sodium ferric gluconate complex in hemodialysis patients: adverse reactions compared to placebo and iron dextran. Kidney Int 61:1830–1839
Bastani B, Rahman S, Gellens M (2002) Lack of reaction to ferric gluconate in hemodialysis patients with a history of severe reaction to iron dextran. ASAIO J 48(4):404–406
Nissenson AR, Lindsay RM, Swan S, Seligman P, Strobos JD (1999) Sodium ferric gluconate complex in sucrose is safe and effective in hemodialysis patients: North American clinical trial. Am J Kidney Dis 33:471–482
Coyne DW, Adkinson NF, Nissenson AR, Fishbane S, Agarwal R, Eschbach JW, Michael B, Folkert V, Batlle D, Trout JR, Dahl N, Myirski P, Strobos J, Warnock DG; Ferrlecit Investigators (2003) Sodium ferric gluconate complex in hemodialysis patients. II. Adverse reactions in iron dextran-sensitive and dextran-tolerant patients. Kidney Int 63:217–224
Michael B, Coyne DW, Folkert VW, Dahl NV, Warnock DG; Ferrlecit Publication Committee (2004) Sodium ferric gluconate complex in haemodialysis patients: a prospective evaluation of long-term safety. Nephrol Dial Transplant 19:1576–1580
Van Wyck DB, Cavallo G, Spinowitz BS, Adhikarla R, Gagnon S, Charytan C, Levin N (2000) Safety and efficacy of iron sucrose in patients sensitive to iron dextran: North American clinical trial. Am J Kidney Dis 36:88–97
Aronoff GR, Bennett WM, Blumenthal S, Charytan C, Pennell JP, Reed J, Rothstein M, Strom J, Wolfe A, Van Wyck D, Yee J; United States Iron Sucrose (Venofer) Clinical Trials Group (2004) Iron sucrose in hemodialysis patients: safety of replacement and maintenance regimens. Kidney Int 66:1193–1198
Ferrlecit package insert (2004) Corona, CA, Watson Pharma
Warady BA, Zobrist RH, Wu J, Finan E; The Ferrlecit Pediatric Study Group (2005) Sodium ferric gluconate complex therapy in anemic children on hemodialysis. Pediatr Nephrol 20:1320–1327
Warady BA, Kausz A, Lerner G, Brewer ED, Chadha V, Brugnara C, Dahl NV, Watkins SL (2004) Iron therapy in the pediatric hemodialysis population. Pediatr Nephrol 19:655–661
Tenbrock K, Muller-Berhaus J, Michalk D, Querfeld U (1999) Intravenous iron treatment of renal anemia in children on hemodialysis. Pediatr Nephrol 13:580–582
Ruiz-Jaramillo M, Guizar-Mendoza JM, Gutierrez-Navarro M, Dubey-Ortega LA, Amador-Licona N (2004) Intermittent versus maintenance iron therapy in children on hemodialysis: a randomized study. Pediatr Nephrol 19:77–81
Morgan HE, Gautam M, Geary DF (2001) Maintenance intravenous iron therapy in pediatric hemodialysis patients. Pediatr Nephrol 16:779–783
Leijn E, Monnens LA, Cornelissen EA (2004) Intravenous iron supplementation in children on hemodialysis. J Nephrol 17:423–426
Besarab A, Kaiser JW, Frinak S (1999) A study of parenteral iron regimens in hemodialysis patients. Am J Kidney Dis 34:21–28
Bolanos L, Castro P, Falcon TG, Mouzo R, Varela JM (2002) Continuous intravenous sodium ferric gluconate improves efficacy in the maintenance phase of EPOrHu administration in hemodialysis patients. Am J Nephrol 22:67–72
Centers for Medicare and Medicaid Services (2004) Annual report, End Stage Renal Disease Clinical Performance Measures Project. Department of Health and Human Services, Centers for Medicare and Medicaid Services, Office of Clinical Standards and Quality, Baltimore
Nailescu C, Castaneda M, Del Rio M, Flynn JT (2004) Iron supplementation in adolescent hemodialysis patients. Clin Nephrol 62:449–454
Yorgin PD, Belson A, Sarwal M, Alexander SR (2000) Sodium ferric gluconate therapy in renal transplant and renal failure patients. Pediatr Nephrol 15:171–175
Kessler M, Hoen B, Mayeux D, Hestin D, Fontenaille C (1993) Bacteremia in patients on chronic hemodialysis. Nephron 64:95–100
Hoen B, Kessler M, Hestin D, Mayeux D (1995) Risk factors for bacterial infections in chronic haemodialysis adult patients: a multicentre prospective survey. Nephrol Dial Transplant 10:377–381
Hoen B, Paul-Dauphin A, Hestin D, Kessler M (1998) EPIBACDIAL: a multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J Am Soc Nephrol 9:869–876
Acknowledgements
The authors wish to acknowledge the contributions of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) organization.
Ferrlecit Pediatric Study Group: Nadine M. Benador, University of California, San Diego, USA; Mark R. Benfield, University of Alabama at Birmingham, USA; Joseph T. Flynn, Montefiore Medical Center, USA; William E. Harmon, Children’s Hospital of Boston, USA; Gary R. Lerner, Children’s Hospital - Los Angeles, USA; Isidro B. Salusky, UCLA School of Medicine, USA; Mouin G. Seikaly, University of Texas Southwestern Medical Center, USA; Barry L. Warshaw, Children’s Healthcare of Atlanta at Egleston, USA; Sandra L. Watkins, Children’s Hospital and Regional Medical Center, USA; Peter D. Yorgin, Stanford University Medical Center, USA.
This trial was supported by a grant from Watson Laboratories to each of the participating centers. This work was presented in part at the 2005 Annual Meeting at the American Society of Nephrology.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Warady, B.A., Zobrist, R.H., Finan, E. et al. Sodium ferric gluconate complex maintenance therapy in children on hemodialysis. Pediatr Nephrol 21, 553–560 (2006). https://doi.org/10.1007/s00467-006-0042-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-006-0042-5