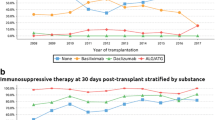
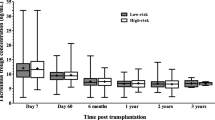
Abstract
Tailored immunosuppression according to risk stratification for optimal outcome for both immunological and non-immunological risk factors should be the ultimate objective for every child in whom renal transplantation is planned. Renal allograft survival is dependent on the appropriate use of immunosuppressive therapy to prevent acute rejection and chronic allograft nephropathy. Unfortunately, all immunosuppressive therapies, including corticosteroids, have unwanted side effects, including infections, malignancy, nephrotoxicity, hypertension, hyperlipidaemia and diabetes mellitus. However, the most worrying side effects of corticosteroids for children, adolescents and their parents are growth retardation and the cosmetic effects. Consequently, achieving immunosuppressive regimens without corticosteroids would be preferable. The major concern for paediatric nephrologists in the 21st century is no longer acute rejection, as the incidence appears to be decreasing, but infection, particularly EBV and the development of post-transplant lymphoproliferative disease (PTLD). With modern immunosuppressive agents in transplantation, rejection is being traded for infection. The long-term outcome data of PTLD with steroid-free and monoclonal antibody protocols is as yet unknown.
Similar content being viewed by others
References
Karin M (1998) New twists in gene regulation by glucocorticoid receptor: is DNA binding dispensable? Cell 93:487–490
Birkeland SA, Larsen KE, Rohr N (1998) Pediatric renal transplantation without steroids. Pediatr Nephrol 12:87–92
Chakrabarti P, Wong HY, Scantlebury VP, Jordan ML, Vivas C, Ellis D, Lombardozzi-Lane S, Hakala TR, Fung JJ, Simmons RL, Starzl TE, Shapiro R (2000) Outcome after steroid withdrawal in pediatric renal transplant patients receiving tacrolimus-based immunosuppression. Transplantation 70:760–764
Roberti I, Reisman L, Lieberman KV, Burrows L (1994) Risk of steroid withdrawal in pediatric renal allograft recipients (a 5-year follow-up). Clin Transplant 8:405–408
North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) 2005 Annual Report (2005) http://spitfire.emmes.com/study/ped/resources/annlrept2005.pdf, 1–266 Accessed online on 16 October 2005. Ref Type: Generic
Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC (2005) Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. BMJ 331:810
Hymes LC, Warshaw BL (2005) Sirolimus in pediatric patients: results in the first 6 months post-renal transplant. Pediatr Transplant 9:520–522
Benfield MR, McDonald RA, Bartosh S, Ho PL, Harmon W (2003) Changing trends in pediatric transplantation: 2001 Annual Report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Transplant 7:321–335
Dharnidharka VR, Sullivan EK, Stablein DM, Tejani AH, Harmon WE (2001) Risk factors for posttransplant lymphoproliferative disorder (PTLD) in pediatric kidney transplantation: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). Transplantation 71:1065–1068
Funch DP, Brady J, Ko HH, Dreyer NA, Walker AM (2002) Methods and objectives of a large US multicenter case-control study of post-transplant lymphoproliferative disorder in renal transplant patients. Recent Results Cancer Res 159:81–88
Rhen T, Cidlowski JA (2005) Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med 353:1711–1723
Ortiz PA, Garvin JL (2003) Cardiovascular and renal control in NOS-deficient mouse models. Am J Physiol Regul Integr Comp Physiol 284:R628–R638
Pape L, Ehrich JH, Zivicnjak M, Offner G (2005) Growth in children after kidney transplantation with living related donor graft or cadaveric graft. Lancet 366:151–153
Fine RN, Stablein D (2005) Long-term use of recombinant human growth hormone in pediatric allograft recipients: a report of the NAPRTCS Transplant Registry. Pediatr Nephrol 20:404–408
Nissel R, Brazda I, Feneberg R, Wigger M, Greiner C, Querfeld U, Haffner D (2004) Effect of renal transplantation in childhood on longitudinal growth and adult height. Kidney Int 66:792–800
Sarwal M (2005) Steroid elimination is coming of age. Pediatr Nephrol 21:2–4 DOI:10.1007/s00467-005-1042-2
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marks, S.D., Trompeter, R.S. Steroid preservation: the rationale for continued prescribing. Pediatr Nephrol 21, 305–307 (2006). https://doi.org/10.1007/s00467-005-2155-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-005-2155-7