Abstract
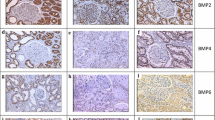
Bone morphogenetic protein 7 (BMP 7) is a member of the transforming growth factor (TGF) beta superfamily and is involved in regeneration, repair, and development of specific tissues, for example kidney, gut, lens, and skeleton. BMP 7 has emerged as a renotrophic factor and experimental studies have shown its protective role against fibrotic processes. Tubulointerstitial changes are present in the pyelonephritic kidney which progresses to fibrosis. Renal fibrosis may lead to significant morbidity in the form of hypertension, proteinuria, and loss of renal function. The objective of this study was to investigate BMP 7 expression in experimental acute and chronic pyelonephritis models. Eighteen Wistar rats were injected with 0.1 mL solution containing E. coli ATCC 25922 1010 cfu mL−1 into left renal medullae. Six rats were used as a sham group and were given 0.1 mL 0.9% NaCl. Pyelonephritic rats were sacrificed 24 h (group I, n=6), 1 week (group II, n=6), and 6 weeks (group III, n=6) after E. coli injection. Serum creatinine levels were analyzed. Renal tissues were studied histopathologically by use of hematoxylin and eosin and scored for diagnosis of pyelonephritis. BMP 7 expression was studied semiquantitatively by immunohistochemical staining. Acute (group I) and chronic (group II and group III) pyelonephritic histopathological changes were observed in experimental pyelonephritic groups. A gradual decrease in BMP 7 expression was observed in the tubulointerstitial and tubular area of the pyelonephritic kidneys, mildest in the acute pyelonephritic group and most severe in the chronic pyelonephritic 6th week group. A statistically significant difference was observed between tubulointerstitial BMP 7 expression by groups I and III (P=0.017) and by groups III and IV (P=0.000). Tubular BMP 7 expression was statistically significantly different between groups II and IV (P=0.009) and between groups III and IV (P=0.002). The data imply that BMP 7 has a major role in chronic pyelonephritis. Tubulointerstitial and tubular BMP 7 expression also had a significant negative correlation with fibrosis, tubular, atrophy, and vascular changes. Serum creatinine levels of the study group were all normal. We conclude that the decrease in renal BMP 7 expression in experimental chronic pyelonephritis is one of the factors responsible for fibrotic changes in persistent renal damage.


Similar content being viewed by others
References
Rushton HG, Majd M, Jantausch B, Wiedermann BL, Belman AB (1992) Renal scarring following reflux and nonreflux pyelonephritis in children: evaluation with 99mTechnetium dimercaptosuccinic acid (DMSA) scintigraphy. J Urol 147:1327
Sirin A, Emre S, Alpay H, Nayır A, Bilge I, Tanman F (1995) Etiology of chronic renal failure in Turkish children. Pediatr Nephrol 9(5):549–552
Heptinstall RH (1992) Pyelonephritis: pathologic features. In Heptinstall RD (eds) Pathology of the kidney, 4th edn. Little Brown, Boston, pp 1489–1561
Wolfman NM, Hattesley G, Cox K (1997) Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7 numbers of the TGF-beta gene family. J Clin Invest 100:321–330
Kim RY, Robertson EJ, Solloway MJ (2001) Bmp6 and Bmp7 are required for cushion formation and septation in the developing mouse heart. Dev Biol 235:449–466
Jena N, Martin-Seisdedos C, McCue P, Croce CM (1997) BMP7 null mutation in mice: developmental defects in skeleton, kidney, and eye. Exp Cell Res 230:28–37
Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G (1995) BMP 7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev 9:2808–2820
Dudley AT, Lyons KM, Robertson EJ (1995) A requirement for bone morphogenetic protein-7 during development of mammalian kidney and eye. Genes Dev 9:2795–2807
Simon M, Maresh JG, Harris SE, Hernandez JD, Arar M, Olson MS, Abboud HE (1999) Expression of bone morphogenetic protein-7 mRNA in normal and ischemic adult rat kidney. Am J Physiol 276: F382–F389
Gould SE, Day M, Jones SS, Dorai H (2002) BMP-7 regulates chemokine, cytokine, and hemodynamic gene expression in proximal tubule cells. Kidney Int 61:51–60
Vukicevic S, Basic V, Rogic D, Basic N, Shih MS, Shepard A, Jin D, Dattatreyamurty B, Jones W, Dorai H, Ryan S, Griffiths D, Maliakal J, Jelic M, Pastorcic M, Stavljenic A, Sampath TK (1998) Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J Clin Invest 102:202–214
Hruska K, Guo G, Wozniak M, Martin D, Miller S, Liapis H, Loveday K, Klahr S, Sampath TK, Morrissey J (2000) Osteogenic protein-1 prevents renal fibrogenesis associated with ureteral obstruction. Am J Physiol 279: F130–F143
Wang S, Chen O, Simon TC, Strebeck F, Chaudhary L, Morrissey J, Liapis H, Klahr S, Hruska KA (2003) Bone morphogenic protein 7 (BMP- 7), a novel therapy for diabetic nephropathy. Kidney Int 63 (6):2037–2349
Almanzar MM, Frazier KS, Dube PH, Piqueras AI, Jones WK, Charette MF, Paredes AL (1998) Osteogenic protein-1 mRNA expression is selectively modulated after acute ischemic renal injury. J Am Soc Nephrol 9 (8):1456–1463
Haraoka M, Matsumoto T, Takahashi K, Kubo S, Tanaka M, Kumazawa J (1994) Supression of renal scarring by prednisolone combined with ciprofloxacin in ascending pyelonephritis in rats. J Urol 151:1078–1080
Kavukcu S, Soylu A, Turkmen M, Sarıoglu S, Buyukgebiz B, Gure A (1999) The role of vitamin A in preventing renal scarring secondary to pyelonephritis. BJU Int 83:1055–1059
Tokunaga S, Ohkawa M, Nakamura S (1993) An experimental model of ascending pyelonephritis due to Candida albicans in rats. Mycopathologia 123:149–154
Androulakakis PA, Ransley PG, Risdon RA, Sorger K, Hohenfellner R (1987) Microvascular changes in the early stage of reflux pyelonephritis. An experimental study in the pig kidney. Eur Urol 13 (4):219–223
Morrissey J, Hruska K, Guo G, Wang S, Chen O, Klahr S (2002) Bone morphogenetic protein-7 (BMP-7) improves renal fibrosis and accelerates the return of renal function. J Am Soc Nephrol 13 (suppl):S14–S21
Kalluri R, Zeisberg M (2003) Exploring the connection between chronic renal fibrosis and bone morphogenetic protein-7. Histol Histopathol 18(1):217–224
Reddi AH (2000) Bone morphogenetic proteins and skeletal development: the kidney bone connection. Pediatr Nephrol 14:598–601
Brock 3rd J, Hunley T, Adams M, Kon V (1998) Role of the renin-angiotensin system in disorders of the urinary tract. J Urol 160:1812–1819
Border WA, Noble NA (1998) Interactions of transforming growth factor-beta and angiotensin II in renal fibrosis. Hypertension 31:181–188
Ma LJ, Yang H, Gaspert A, Carlesso G, Barty MM, Davidson JM, Sheppard D, Fogo AB (2003) Transforming growth factor-beta-dependent and independent pathways of induction of tubulointerstitial fibrosis in beta 6 (-/-) mice. Am J Pathol 163 (4):1261–1273
Yamashita S, Maeshima A, Kojima I, Nojima Y (2004) Activin A is a potent activator of renal interstitial fibrosis. J Am Soc Nephrol 15(1):91–101
Negri Al (2004) Prevention of progressive fibrosis in chronic renal diseases: antifibrotic agents. J Nephrol 17(4):496–503
Khalil A, Tullus K, Bakhiet M, Burman LG, Jaremko G, Brauner A (2000) Angiotensin II type I receptor antagonist (losartan) down-regulates transforming growth factor-beta in experimental acute pyelonephritis. J Urol 164 (1):186–191
Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charyton D, Strutz F, Kalluri R (2003) BMP 7 counteracts TGF-b1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9:964–968
Wang SN, Lapage J, Hirschberg R (2001) Loss of bone morphogenetic protein-7 in diabetic nephropathy. J Am Soc Nephrol 12:2392–2399
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Biyikli, N.K., Tugtepe, H., Cakalagaoglu, F. et al. Downregulation of the expression of bone morphogenetic protein 7 in experimental pyelonephritis. Pediatr Nephrol 20, 1230–1236 (2005). https://doi.org/10.1007/s00467-005-1927-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-005-1927-4