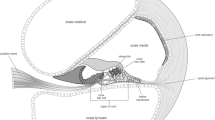
Abstract
Inner ear physiology is reviewed with emphasis on features common to renal physiology. Genetic disorders in transporters/channels for chloride (ClC-K), bicarbonate (Cl-/HCO3- exchanger), protons (H+-ATPase), sodium (ENaC, NKKC1, NBC3, NHE3), potassium (KCNQ1/KCNE1, Kcc4), and water (AQP4) in the inner ear and their relation to the kidney are discussed. Based on data from human disorders (with or without mouse counterparts) and mouse models (without human counterparts) this article focuses on the involvement of these transporters/channels in hearing loss.






Similar content being viewed by others
References
Willems PJ (2000) Genetic causes of hearing loss. N Engl J Med 342:1101–1109
Tekin M, Arnos KS, Pandya A (2001) Advances in hereditary deafness. Lancet 358:1082–1090
Couloigner V, Sterkers O, Friedlander G, Ferrary E (2003) Syndromes ‘reins-oreilles’: aspects moléculaires. Actualités Néphrolologiques Flammarion, Paris, pp 147–161
Ferrary E, Sterkers O (1998) Mechanisms of endolymph secretion. Kidney Int 65:98–103
Salt AN (2001) Dynamics of the inner ear fluids. In: Jahn AF, Santos-Sacchi J (eds) Physiology of the ear. Singular, San Diego, pp 333–355
Wangemann P (2002) K+ cycling and its regulation in the cochlea and the vestibular labyrinth. Audiol Neurootol 7:199–205
Peters TA. Kuijpers W, Tonnaer ELGM, Muijen van GNP, Jap PHK (1995) Distribution and features of melanocytes during inner ear development in pigmented and albino rats. Hear Res 85:169–180
Peters TA, Kuijpers W, Curfs JHAJ (2001) Occurrence of NaK-ATPase isoforms during rat inner ear development and functional implications. Eur Arch Otorhinolaryngol 258:67–73
Sage CL, Marcus DC (2001) Immunolocalization of ClC-K chloride channel in strial marginal cells and vestibular dark cells. Hear Res 160:1–9
Estevez R, Boettger T, Stein V, Birkenhager R, Otto E, Hildebrandt F, Jentsch TJ (2001) Barttin is a Cl- channel β subunit crucial for renal Cl- reabsorption and inner ear K+ secretion. Nature 414:558–561
Couloigner V, Fay M, Djelidi S, Farman N, Escoubet B, Runembert I, Sterkers O, Friedlander G, Ferrary E (2001) Location and function of the epithelial Na channel in the cochlea. Am J Physiol Renal Physiol 280:F214–F222
Bond BR, Ng LL, Schulte BA (1998) Identification of mRNA transcripts and immunohistochemical localization of Na/H exchanger isoforms in gerbil inner ear. Hear Res 123:1–9
Stankovic KM, Brown D, Alper SL, Adams JC (1997) Localization of pH regulating proteins H+-ATPase and Cl-/HCO3- exchanger in the guinea pig inner ear. Hear Res 114:21–34
Marcus DC, Wu T, Wangemann P, Kofuji P (2002) KCNJ10 (Kir4.1) potassium channel knockout abolishes endocochlear potential. Am J Physiol Cell Physiol 282:C403–C407
Wangemann P (2002) K+ cycling and the endocochlear potential. Hear Res 165:1–9
Waldegger S, Jeck N, Bart P, Peters M, Vizthum H, Wolf K, Kurtz A, Konrad M, Seyberth HW (2002) Barttin increases surface expression and changes current properties in ClC-K channels. Pflug Arch Eur J Physiol 444:411–418
Uchida S, Maromo F (2000) Severity impaired urine-concentrating ability in mice lacking the ClC-K1 chloride channel. Exp Nephrol 8:301–305
Schlingmann KP, Konrad M, Jeck N, Waldegger P, Reinalter SC, Holder M, Seyberth HW, Waldegger S (2004) Salt wasting and deafness resulting from mutations in two chloride channels. N Engl J Med 350:1314–1319
Wall SM, Hassell KA, Royaux IE, Green ED, Chang JY, Shipley GL, Verlander JW (2003) Localization of pendrin in mouse kidney. Am J Physiol Renal Physiol 284:F229–F241
Petrovic S, Wang Z, Ma L, Soleimani M (2003) Regulation of the apical Cl-/HCO3- exchanger pendrin in rat collecting duct in metabolic acidosis. Am J Physiol Renal Physiol 284:F103–F112
Royaux I, Suzuki K, Mori A, Katoh R, Everett LA, Kohn LD, Green ED (2000) Pendrin, the protein encoded by the Pendred syndrome gene (PDS), is an apical porter of iodide in the thyroid and is regulated by thyroglobulin in FRTL-5 cells. Endocrinology 141:839–845
Everett LA, Morsli H, Wu DK, Green ED (1999) Expression pattern of the mouse ortholog of the Pendred’s syndrome gene (Pds) suggest a key role for pendrin in the inner ear. Proc Natl Acad Sci U S A 96:9727–9732
Everett LA, Belyantseva IA, Noben-Trauth K, Cantos R, Chen A, Thakkar SI, Hoogstraten-Miller SL, Kachar B, Wu DK, Green ED (2001) Targeted disruption of mouse Pds provides insight about the inner-ear defects encountered in Pendred syndrome. Hum Mol Genet 10:153–161
Karet FE (2002) Inherited distal renal tubular acidosis. J Am Soc Nephrol 13:2178–2184
Karet FE, Finberg KE, Nelson RD, Nayir A, Mocan H, Sanjad SA, Rodriguez-Soriano J, Santos F, Cremers CWRJ, Di Pietro A, Hoffbrand BI, Winiarski J, Bakkaloglu A, Ozen S, Dusunsel R, Goodyer P, Hulton SA, Wu DK, Skovorak AB, Morton CC, Cunningham MJ, Jha V, Lifton RP (1999) Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat Genet 21:84–90
Stover EH, Borthwick KJ, Bavalia C, Eady N, Fritz DM, Rungroj N, Giersch ABS, Morton CC, Axon PR, Akil I, Al-Sabban EA, Baguley DM, Bianca S, Bakkaloglu A, Bircan Z, Chauveau D, Clermont MJ, Guala A, Hulton SA, Kroes H, Li Volti G, Mir S, Mocan H, Nayir A, Ozen S, Rodriguez-Soriano J, Sanjad SA, Tasic V, Taylor CM, Topaloglu R, Smith AN, Karet FE (2002) Novel ATP6V1B1 and ATP6V0A4 mutations in autosomal recessive distal renal tubular acidosis with new evidence for hearing loss. J Med Genet 39:796–803
Dou H, Friberg K, Cardell EL, Lifton R, Choo D (2003) Mice lacking the B1 subunit of H+-ATPase have normal hearing. Hear Res 180:76–84
Guipponi M, Vuagniaux G, Wattenhofer M, Shibuya K, Vazquez M, Dougherty L, Scamuffa N, Guida E, Okui M, Rossie C, Hancock M, Buchet K, Reymond A, Hummler E, Marzella PL, Kudoh J, Shimizu N, Scott HS, Antonarakis SE, Rossier BC (2002) The transmembrane serine protease (TMPRSS3) mutated in deafness DFNB8/10 activates the epithelial sodium channel (ENaC) in vitro. Hum Mol Genet 11:2829–2836
Lee YJ, Park D, Kim SY, Park WJ (2003) Pathogenic mutations but no polymorphisms in congenital and childhood onset autosomal recessive deafness disrupt the proteolytic activity of TMPRSS3. J Med Genet 40:629–631
Neyroud N, Tesson F, Denjoy I, Leibovici M, Donger C, Barhanin J, Faure S, Gary F, Coumel P, Petit C, Schwartz K, Guicheney P (1997) A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange-Nielsen cardioauditory syndrome. Nat Genet 15:186–189
Casimiro MC, Knollmann BV, Ebert SN, Vary JC Jr, Greene AE, Franz MR, Grinberg A, Huang SP, Pfeifer K (2001) Targeted disruption of the kcnq1 gene produces a mouse model of Jervell and Lange-Nielsen syndrome. Proc Natl Acad Sci U S A 98:2526–2531
Warth R, Barhanin J (2002) The multifaceted phenotype of the knockout mouse for the KCNE1 potassium channel gene. Am J Physiol Regul Integr Comp Physiol 282:R639–R648
Tyson J, Tranebjaerg L, Bellman S, Wren C, Taylor JFN, Bathen J, Sorland SJ, Lund O, Malcolm S, Pembrey M, Bhattacharya S, Bitner-Glindzicz M (1997) IsK and KvLQT1: mutation in either of the two subunits of the slow component of the delayed rectifier potassium channel can cause Jervell and Lange-Nielsen syndrome. Hum Mol Genet 6:2179–2185
Russell JM (2000) Sodium-potassium-chloride cotransport. Physiol Rev 80:211–276
Delpire E, Lu J, England R, Dull C, Thorne T (1999) Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat Genet 22:192–195
Rybak LP (1993) Ototoxicity of loop diuretics. Otolaryngol Clin North Am 26:829–844
Wall SM, Fischer MP, Metha P, Hassell KA, Park SJ (2001) Contribution of the Na+-K+-2Cl- cotransporter NKCC1 to Cl- secretion in rat OMCD. Am J Physiol Renal Physiol 280:F913–F921
Bok D, Galbraith G, Lopez, Woodruff M, Nusinowitz S, BeltrandelRio H, Huang W, Zhao S, Geske R, Montgomery C, Sligtenhorst I van, Friddle C, Platt K, Sparks MJ, Pushkin A, Abuladze N, Ishiyama A, Dukkipati R, Liu W, Kurtz I (2003) Blindness and auditory impairment caused by loss of the sodium bicarbonate cotransporter NBC3. Nat Genet 34:313–319
Pushkin A, Yip KP, Clark I, Abuladze N, Kwon TH, Tsuruoka S, Schwartz GJ, Nielsen S, Kurtz I (1999) NBC3 expression in rabbit collecting duct; colocalization with vacuolar H+-ATPase. Am J Physiol Renal Physiol 46:F974–F981
Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull G (1998) Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19:282–285
Boettger T, Hubner CA, Maier H, Rust MB, Beck FX, Jentsch TJ (2002) Deafness and renal tubular acidosis in mice lacking the K-Cl co-transporter Kcc4. Nature 416:874–878
Li J, Verkman AS (2001) Impaired hearing in mice lacking aquaporin-4 water channels. J Biol Chem 278:31233–31237
Rotig A, Munnich A (2003) Genetic features of mitochondrial respiratory chain disorders. J Am Soc Nephrol 14:2995–3007
Street VA, McKee-Johnson JW, Fonseca RC, Tempel BL, Noben-Trauth K (1998) Mutations in a plasma membrane Ca2+-ATPase gene cause deafness in deafwaddler mice. Nat Genet 19:390–394
Mhatre AN, Jero J, Chiappini I, Bolasco G, Barbara M, Lalwani AK (2002) Aquaporin-2 expression in the mammalian cochlea and investigation of its role in Meniere’s disease. Hear Res 170:59–69
Sterkers O, Ferrary E, Amiel C (1984) Inter-and intracompartmental osmotic gradients within the rat cochlea. Am J Physiol 247:F602–F606
Lin J, Ozeki M, Javel E, Zhao Z, Pan W, Schlentz E, Levine S (2003) Identification of gene expression profiles in rat ears with cDNA microarrays. Hear Res 175:2–13
Abe S, Katagiri T, Saito-Hisaminato A (2003) Identification of CRYM as a candidate responsible for nonsyndromic deafness, through cDNA microarray analysis of human cochlear and vestibular tissues. Am J Hum Genet 72:73–82
Peters TA, Tonnaer ELGM, Kuijpers W, Cremers CWRJ, Curfs JHAJ (2002) Differences in endolymphatic sac mitochondria-rich cells indicate specific functions. Laryngoscope 112:534–541
Peters TA, Tonnaer ELGM, Kuijpers W, Curfs JHAJ (2003) Changes in ultrastructural characteristics of endolymphatic sac ribosome-rich cells of the rat during development. Hear Res 176:94–104
Couloigner V, Teixeira M, Sterkers O, Rask-Andersen H, Ferrary E (2004) The endolymphatic sac: its role in the inner ear. Med Sci (Paris) 20:304–310
Ishimoto S, Kawamoto K, Kanzaki S, Raphael Y (2002) Gene transfer into supporting cells of the organ of Corti. Hear Res 173:187–197
Han D, Yu Z, Fan E, Liu C, Liu S, Li Y, Liu Z (2004) Morphology of auditory hair cells in guinea pig cochlea after transgene expression. Hear Res 190:25–30
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peters, T.A., Monnens, L.A.H., Cremers, C.W.R.J. et al. Genetic disorders of transporters/channels in the inner ear and their relation to the kidney. Pediatr Nephrol 19, 1194–1201 (2004). https://doi.org/10.1007/s00467-004-1626-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-004-1626-6