Abstract
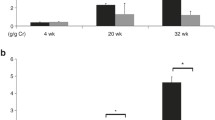
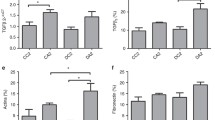
The newborn rat kidney is not fully developed until approximately 12 days after birth. Several lines of evidence suggest that angiotensin II (AII) participates in the postnatal development of the kidney. The aim of the present study was to analyze proliferating cell nuclear antigen (PCNA), fibronectin, α-smooth muscle-actin (α-SM-actin), and AII expression in renal cortex during development in rats born to mothers that received a normal (control) or increased (experimental) sodium intake during pregnancy. Ninety Wistar rats aged 1, 7, 15, and 30 days from the control and experimental groups were killed and the kidneys removed for histological and immunohistochemical studies. The results showed higher fibronectin, α-SM-actin, PCNA, and AII expression in the glomerular and tubulointerstitial areas of the renal cortex of 1- and 7-day-old animals, which decreased with renal development. The animals from the experimental group showed at 1 day of age a decrease in α-SM-actin, fibronectin, PCNA, and AII expression compared with controls of the same age ( P <0.05). In conclusion, our data show that increased sodium intake during pregnancy induces a reduction of α-SM-actin, fibronectin, and PCNA expression in the renal cortex tubulointerstitium and glomeruli of neonatal rats. These alterations may be related to the decrease of AII expression also observed in the renal cortex from these animals.




Similar content being viewed by others
References
Nigam SK, Aperia AC, Brenner BM (1996) Development and maturation of the kidney. In: Brenner BM, Rector FC (eds): The kidney. Saunders, Philadelphia, pp 72–98
Reeves W, Caulfield JP, Farquhar MG (1978) Differentiation of epithelial foot processes and filtration slits: sequential appearance of occluding junctions, epithelial polyanion, and slit membranes in developing glomeruli. Lab Invest 39:90–100
Roberts AB, McCune BK, Sporn MB (1992) TGF-β: regulation of extracellular matrix. Kidney Int 41:557–559
Thiery JP, Duband JL, Dufour S, Savagner P, Imhof BA (1989) Role of fibronectin in embryogenesis. In: Mosher DF (ed) Biology of extracellular matrix: fibronectin. Academic Press, San Diego, pp 181–212
Kagami S, Border WA, Miller DE, Noble NA (1994) Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-β expression in rat glomerular mesangial cells. J Clin Invest 93:2431–2437
Bagby SP, Kirk EA, Mitchell LH, O’Reilly MM, Holden WE, Stenberg PE, Bakke AG (1993) Proliferative synergy of angiotensin II and EGF in porcine aortic vascular smooth muscle cells. Am J Physiol 265:F239–F249
Fernandez LA, Twickler J, Mead A (1985) Neovascularization produced by angiotensin II. J Lab Clin Med 105:141–145
Gomez RA (1990) Molecular biology of components of the renin-angiotensin system during development. Pediatr Nephrol 4:421–423
Gomez RA, Lynch KR, Sturgill BC, Elwood JP, Chevalier RL, Carey RM, Peach MJ (1989) Distribution of renin mRNA and its protein in the developing kidney. Am J Physiol 257: F850–F858
Millan MA, Carvallo P, Izumi S, Zemel S, Catt KJ, Aguilera G (1989) Novel sites of expression of functional angiotensin II receptors in the late gestation fetus. Science 244:1340–1342
Tufro-McReddie A, Harrison JK, Everett AD, Gomez RA (1993) Ontogeny of type 1 angiotensin II receptor gene expression in the rat. J Clin Invest 91:530–537
Grady EF, Sechi LA, Griffin CA, Schambelan M, Kalinyak JE (1991) Expression of AT2 receptors in the developing rat fetus. J Clin Invest 88:921–933
Du Y, Yao A, Guo D, Inagami T, Wang DH (1995) Differential regulation of angiotensin II receptors subtypes in rat kidney by low dietary sodium. Hypertension 25:872–877
Coimbra TM, Janssen U, Gröne HJ, Ostendorf T, Kunter U, Schmidt H, Brabant G, Floege J (2000) Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney Int 57:167–182
Kliem V, Johnson RJ, Alpers CE, Yoshimura A, Couser WG, Koch KM, Floege J (1996) Mechanisms involved in the pathogenesis of tubulointerstitial fibrosis in 5/6-nephrectomized rats. Kidney Int 49:666–678
Stambe C, Atkins RC, Hill PA, Nikolic-Paterson DJ (2003) Activation and cellular localization of the p38 and JNK MAPK pathways in rat crescentic glomerulonephritis. Kidney Int 64:2121–2132
Hartree EF (1972) Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem 48:422–427
Fuehr Y, Kaczmarczk Y, Kruttgen GD (1955) Eine einfache colorimetrische Methode zur Inulin-Bestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr 33:729–730
Alpers CE, Hudkins KL, Gown AM, Johnson RJ (1992) Enhanced expression of “muscle-specific” actin in glomerulonephritis. Kidney Int 41:1134–1142
El Nahas AM, Muchaneta-Kubara EC, Zhang G, Adam A, Goumenos D (1996) Phenotypic modulation of renal cells during experimental and clinical renal scarring. Kidney Int 54:S23–S27
Geleilete TJ, Melo GC, Costa RS, Volpini RA, Soares TJ, Coimbra TM (2002) Role of myofibroblasts, macrophages, transforming growth factor-beta, endothelin, angiotensin-II, and fibronectin in the progression of tubulointerstitial nephritis induced by gentamicin. J Nephrol 15:633–642
Johnson RJ, Alpers CE, Yoshimura A, Lombardi D, Pritzl P, Floege J, Schwartz SM (1992) Renal injury from angiotensin II-mediated hypertension. Hypertension 19:464–474
Chen Y, Lasaitiene D, Gabrielsson BG, Carlsson LM, Billig H, Carlsson B, Marcussen N, Sun XF, Friberg P (2004) Neonatal losartan treatment suppresses renal expression of molecules involved in cell-cell and cell-matrix interactions. J Am Soc Nephrol 15:1232–1243
Johnson RJ, Iida H, Alpers CE, Majesky MW, Schwartz SM, Pritzl P, Gordon K, Gown AM (1991) Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest 87:847–858
Carey AV, Carey RM, Gomez RA (1992) Expression of alpha-smooth muscle actin in the developing kidney vasculature. Hypertension 19 [Suppl]:168–175
Naruse K, Fujieda M, Miyasaki E, Hayashi Y, Toi M, Fukui T, Kuroda N, Hiroi M, Kurashige T, Enzan H (2000) An immunohistochemical study of developing glomeruli in human fetal kidneys. Kidney Int 57:1836–1846
Omori S, Hida M, Ishikura K, Kuramochi S, Awazu M (2000) Expression of mitogen-activated protein kinase family in rat renal development. Kidney Int 58:27–37
Hsuch WA, Do YS, Anderson PW, Law RE (1995) Angiotensin II in cell growth and matrix production. Adv Exp Med Biol 377:217–223
McCausland JE, Ryan GB, Alcorn D (1996) Angiotensin converting enzyme inhibition in the postnatal rat results in decreased cell proliferation in the renal outer medulla. Clin Exp Pharmacol Physiol 23:552–554
Guron G, Friberg P (2000) An intact renin angiotensin system is a prerequisite for normal renal development. J Hypertens 18:123–137
Nicolantonio RD, Spargo S, Morgan TO (1987) Prenatal high salt diet increases blood pressure and salt retention in spontaneously hypertensive rat. Clin Exp Pharmacol Physiol 14:233–235
Silva AA, Noronha IL, Oliveira IB de, Malheiros DMC, Heimann JC (2003) Renin-angiotensin system function and blood pressure in adult rats after perinatal salt overload. Nutr Metab Cardiovasc Dis 13:133–139
Hazon N, Parker C, Leonard R, Henderson IW (1988) Influence of an enriched sodium chloride regime during gestation and suckling and post-natally on the ontogeny of hypertension in the rat. J Hypertens 6:517–524
Acknowledgements
The authors thank Cleonice G.A. da Silva, Erika Delloiagono, and Rubens Fernando de Melo for expert technical assistance. Ana Paula C. Balbi was a recipient of Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, DF, Brazil, fellowship, and Dr. Roberto Silva Costa and Dr. Terezila Machado Coimbra are recipients of Conselho Nacional de Desenvolvimento Científico e Tecnológico, DF, Brazil, fellowships. These results were presented in abstract form at the American Society of Nephrology Meeting, Philadelphia, Pa., November 2002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balbi, A.P.C., Costa, R.S. & Coimbra, T.M. Postnatal renal development of rats from mothers that received increased sodium intake. Pediatr Nephrol 19, 1212–1218 (2004). https://doi.org/10.1007/s00467-004-1586-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-004-1586-x