Abstract
Purpose
Helicobacter pylori (HP) is the most common human infection that has affected up to 50% of the population worldwide. The relationship between HP eradication and weight loss is under debate. The present study aimed to compare weight loss outcomes after Roux-en-Y gastric bypass (RYGB) in HP-negative (HP−) and HP-eradicated (HPe) patients during five years follow-ups.
Methods
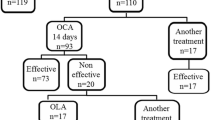
This retrospective cohort study was conducted on 305 patients aged 18 and more with severe obesity, who underwent primary RYGB from February 2014 to November 2017. The HP-negative and HP-eradicated patients were evaluated for weight loss outcomes during five years follow-ups.
Results
Patients' mean age, mean weight, and mean body mass index were 38.78 ± 9.9, 114.8 ± 13.6, and 43.37 ± 2.55, respectively. 27.2% of patients who were HP-positive were treated before RYGB. There was no significant difference between the HP− and HPe patients in terms of total weight loss percent (%TWL), 12 to 60 months after RYGB. Excess weight loss percent (%EWL) was higher in HPe patients compared to HP− patients (P = 0.04) at 12-month after RYGB. However, there was no difference in %EWL between these two groups of patients, 36 and 60 months after RYGB.
Conclusion
The results of the present study showed that TWL% had no significant difference in HP− and HPe groups during five years follow-ups after RYGB. The %EWL was higher in HPe patients only at 12 months after RYGB and the difference did not persist over time.
Graphical Abstract

Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Brown LM (2000) Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev 22(2):283–297
Mocanu V, Dang JT, Switzer N, Skubleny D, Shi X, de Gara C et al (2018) The effect of Helicobacter pylori on postoperative outcomes in patients undergoing bariatric surgery: a systematic review and meta-analysis. Obes Surg 28:567–573
Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F et al (2012) Management of Helicobacter pylori infection—the Maastricht IV/Florence consensus report. Gut 61(5):646–664
Patel SK, Pratap CB, Jain AK, Gulati AK, Nath G (2014) Diagnosis of Helicobacter pylori: what should be the gold standard? World J Gastroenterol 20(36):12847
Wang Y-K, Kuo F-C, Liu C-J, Wu M-C, Shih H-Y, Wang SS et al (2015) Diagnosis of Helicobacter pylori infection: current options and developments. World J Gastroenterol 21(40):11221
Testerman TL, Morris J (2014) Beyond the stomach: an updated view of Helicobacter pylori pathogenesis, diagnosis, and treatment. World J Gastroenterol 20(36):12781
Chiappetta S, Stier C, Ghanem OM, Dayyeh BKA, Boškoski I, Prager G et al (2023) Perioperative interventions to prevent gastroesophageal reflux disease and marginal ulcers after bariatric surgery - an international experts’ survey. Obes Surg 33(5):1449–1462
Kelly JJ, Perugini RA, Wang QL, Czerniach DR, Flahive J, Cohen PA (2015) The presence of Helicobacter pylori is not associated with long-term anastomotic complications in gastric bypass patients. Surg Endosc 29:2885–2890
Zanotti D, Elkalaawy M, Hashemi M, Jenkinson A, Adamo M (2016) Current status of preoperative oesophago-gastro-duodenoscopy (OGD) in bariatric NHS units—a BOMSS survey. Obes Surg 26:2257–2262
Iwańczak F, Iwańczak B (2012) Treatment of Helicobacter pylori infection in the aspect of increasing antibiotic resistance. Adv Clin Exp Med 21(5):671–680
Monno R, De Laurentiis V, Trerotoli P, Roselli AM, Ierardi E, Portincasa P (2019) Helicobacter pylori infection: association with dietary habits and socioeconomic conditions. Clin Res Hepatol Gastroenterol 43(5):603–607
Baradaran A, Dehghanbanadaki H, Naderpour S, Pirkashani LM, Rajabi A, Rashti R et al (2021) The association between Helicobacter pylori and obesity: a systematic review and meta-analysis of case–control studies. Clin Diabet Endocrinol 7(1):1–11
Hegde V, Dhurandhar N (2013) Microbes and obesity—interrelationship between infection, adipose tissue and the immune system. Clin Microbiol Infect 19(4):314–320
Gunji T, Matsuhashi N, Sato H, Fujibayashi K, Okumura M, Sasabe N et al (2009) Helicobacter pylori infection significantly increases insulin resistance in the asymptomatic Japanese population. Helicobacter 14(5):496–502
Erim T, Cruz-Correa MR, Szomstein S, Velis E, Rosenthal R (2008) Prevalence of Helicobacter pylori seropositivity among patients undergoing bariatric surgery: a preliminary study. World J Surg 32:2021–2025
Carabotti M, D’Ercole C, Iossa A, Corazziari E, Silecchia G, Severi C (2014) Helicobacter pylori infection in obesity and its clinical outcome after bariatric surgery. World J Gastroenterol 20(3):647
Maksud FA, Alves JS, Diniz MT, Barbosa AJ (2011) Density of ghrelin-producing cells is higher in the gastric mucosa of morbidly obese patients. Eur J Endocrinol 165(1):57–62
Arslan E, Atılgan H, Yavaşoğlu İ (2009) The prevalence of Helicobacter pylori in obese subjects. Eur J Intern Med 20(7):695–697
Xu M-Y, Liu L, Yuan B-S, Yin J, Lu Q-B (2017) Association of obesity with Helicobacter pylori infection: a retrospective study. World J Gastroenterol 23(15):2750
Kermansaravi M, Shahmiri SS, Khalaj A, Jalali SM, Amini M, Alamdari NM et al (2022) The first web-based Iranian National Obesity and Metabolic Surgery Database (INOSD). Obes Surg 32(6):2083–2086
Pazouki A, Kermansaravi M (2019) Single Anastomosis Sleeve-Jejunal Bypass: a new method of bariatric/metabolic surgery. Obes Surg 29(11):3769–3770
Kermansaravi M, Parmar C, Chiappetta S, Shikora S, Aminian A, Abbas SI et al (2023) Best practice approach for redo-surgeries after sleeve gastrectomy, an expert’s modified Delphi consensus. Surg Endosc 37(3):1617–1628
Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D et al (2017) Global prevalence of helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 153(2):420–429
Wu M-S, Lee W-J, Wang H-H, Huang S-P, Lin J-T (2005) A case-control study of association of Helicobacter pylori infection with morbid obesity in Taiwan. Arch Intern Med 165(13):1552–1555
Nwokolo C, Freshwater D, O’hare P, Randeva H (2003) Plasma ghrelin following cure of Helicobacter pylori. Gut 52(5):637–640
Carabotti M, D’Ercole C, Iossa A, Corazziari E, Silecchia G, Severi C (2014) Helicobacter pylori infection in obesity and its clinical outcome after bariatric surgery. World J Gastroenterol 20(3):647–653
Smelt HJM, Smulders JF, Gilissen LPL, Said M, Ugale S, Pouwels S (2018) Influence of Helicobacter pylori infection on gastrointestinal symptoms and complications in bariatric surgery patients: a review and meta-analysis. Surg Obes Relat Dis 14(10):1645–1657
Goday A, Castañer O, Benaiges D, Pou AB, Ramón JM, Iglesias MdM, et al (2018) Can Helicobacter pylori eradication treatment modify the metabolic response to bariatric surgery? Obes Surg 28:2386–95
Shanti H, Almajali N, Al-Shamaileh T, Samarah W, Mismar A, Obeidat F (2017) Helicobacter pylori does not affect postoperative outcomes after sleeve gastrectomy. Obes Surg 27:1298–1301
Gomberawalla A, Lutfi R (2015) Early outcomes of helicobacter pylori and its treatment after laparoscopic sleeve gastrectomy. Bariatr Surg Pract Patient Care 10(1):12–14
Brownlee AR, Bromberg E, Roslin MS (2015) Outcomes in patients with Helicobacter pylori undergoing laparoscopic sleeve gastrectomy. Obes Surg 25:2276–2279
Emile SH, Elshobaky A, Elbanna HG, Elkashef W, Abdel-Razik MA (2020) Helicobacter pylori, sleeve gastrectomy, and gastroesophageal reflux disease; is there a relation? Obes Surg 30(8):3037–3045
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
Drs Behnood Farazmand, Masoumeh Shahsavan, Foolad Eghbali, Abdolreza Pazouki and Mohammad Kermansaravi, have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Farazmand, B., Shahsavan, M., Eghbali, F. et al. Comparison of weight loss after Roux-en-Y gastric bypass in Helicobacter pylori-negative and Helicobacter pylori eradicated patients during five years follow-ups. Surg Endosc 38, 888–893 (2024). https://doi.org/10.1007/s00464-023-10578-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10578-w