Abstract
Background
Indocyanine green (ICG) is an injectable fluorochrome that has recently gained popularity as a means of assisting intraoperative visualization during laparoscopic and robotic surgery. Many systematic reviews and meta-analyses have been published. We conducted a meta-review to synthesize the findings of these studies.
Methods
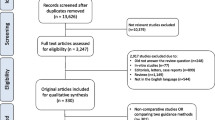
PubMed and Embase were searched to identify systematic reviews and meta-analyses coping with the uses of ICG in abdominal operations, including Metabolic Bariatric Surgery, Cholecystectomy, Colorectal, Esophageal, Gastric, Hepato-Pancreato-Biliary, Obstetrics and Gynecology (OG), Pediatric Surgery, Surgical Oncology, Urology, (abdominal) Vascular Surgery, Adrenal and Splenic Surgery, and Interdisciplinary tasks, until September 2023. We submitted the retrieved meta-analyses to qualitative analysis based on the AMSTAR 2 instrument.
Results
We identified 116 studies, 41 systematic reviews (SRs) and 75 meta-analyses (MAs), spanning 2013–2023. The most thoroughly investigated (sub)specialties were Colorectal (6 SRs, 25 MAs), OG (9 SRs, 15 MAs), and HPB (4 SRs, 12 MAs). Interestingly, there was high heterogeneity regarding the administered ICG doses, routes, and timing. The use of ICG offered a clear benefit regarding anastomotic leak prevention, particularly after colorectal and esophageal surgery. There was no clear benefit regarding sentinel node detection after OG. According to the AMSTAR 2 tool, most meta-analyses ranked as “critically low” (34.7%) or “low” (58.7%) quality. There were only five meta-analyses (6.7%) that qualified as “moderate” quality, whereas there were no “high” quality reviews.
Conclusions
Regardless of the abundance of pertinent literature and reviews, surgeons should be cautious when interpreting their results on ICG use in abdominal surgery. Future reviews should focus on ensuring methodological vigor; establishing clear protocols of ICG dose, route of administration, and timing; and improving reporting quality. Other sources of data (e.g., registries) and novel methods of data analysis (e.g., machine learning) might also contribute to an enhanced role of ICG as a decision-making tool in surgery.
Graphical abstract









Similar content being viewed by others
Change history
12 February 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00464-024-10744-8
12 December 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00464-023-10646-1
References
Cassinotti E et al (2023) European Association for Endoscopic Surgery (EAES) consensus on Indocyanine Green (ICG) fluorescence-guided surgery. Surg Endosc 37(3):1629–1648. https://doi.org/10.1007/s00464-023-09928-5
Mortensen OE, Nerup N, Thorsteinsson M, Svendsen MBS, Shiwaku H, Achiam MP (2020) Fluorescence guided intraluminal endoscopy in the gastrointestinal tract: a systematic review. World J Gastrointest Endosc 12(10):388–400. https://doi.org/10.4253/wjge.v12.i10.388
Zelken JA, Tufaro AP (2015) Current trends and emerging future of indocyanine green usage in surgery and oncology: an update. Ann Surg Oncol 22(Suppl 3):S1271–S1283. https://doi.org/10.1245/s10434-015-4743-5
Xu P-Y, Zheng X, Kankala RK, Wang S-B, Chen A-Z (2021) Advances in indocyanine green-based codelivery nanoplatforms for combinatorial therapy. ACS Biomater Sci Eng 7(3):939–962. https://doi.org/10.1021/acsbiomaterials.0c01644
Page MJ et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Shea BJ et al (2017) AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 358:j4008. https://doi.org/10.1136/bmj.j4008
Cheng S-T, Zhang F (2020) A comprehensive meta-review of systematic reviews and meta-analyses on nonpharmacological interventions for informal dementia caregivers. BMC Geriatr 20(1):137. https://doi.org/10.1186/s12877-020-01547-2
Gillespie BM et al (2020) Preoperative and postoperative recommendations to surgical wound care interventions: a systematic meta-review of Cochrane reviews. Int J Nurs Stud 102:103486. https://doi.org/10.1016/j.ijnurstu.2019.103486
Hsu A, Mu SZ, James A, Ibrahim MA, Saber AA (2023) Indocyanine green in bariatric surgery: a systematic review. Obes Surg. https://doi.org/10.1007/s11695-023-06801-1
Wityk M, Dowgiałło-Gornowicz N, Feszak I, Bobowicz M (2023) Fluorescence use in minimally invasive metabolic and bariatric surgery—a systematic review of the literature. Langenbecks Arch Surg 408(1):216. https://doi.org/10.1007/s00423-023-02955-9
Pesce A, Piccolo G, La Greca G, Puleo S (2015) Utility of fluorescent cholangiography during laparoscopic cholecystectomy: a systematic review. World J Gastroenterol 21(25):7877–7883. https://doi.org/10.3748/wjg.v21.i25.7877
Serban D et al (2022) Systematic review of the role of indocyanine green near-infrared fluorescence in safe laparoscopic cholecystectomy (review). Exp Ther Med 23(2):187. https://doi.org/10.3892/etm.2021.11110
van den Bos J, Wieringa FP, Bouvy ND, Stassen LPS (2018) Optimizing the image of fluorescence cholangiography using ICG: a systematic review and ex vivo experiments. Surg Endosc 32(12):4820–4832. https://doi.org/10.1007/s00464-018-6233-x
Brollo PP et al (2023) Preventing iatrogenic ureteral injury in colorectal surgery: a comprehensive and systematic review of the last 2 decades of literature and future perspectives. Surg Today. https://doi.org/10.1007/s00595-022-02639-9
Liberale G, Bourgeois P, Larsimont D, Moreau M, Donckier V, Ishizawa T (2017) Indocyanine green fluorescence-guided surgery after IV injection in metastatic colorectal cancer: a systematic review. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 43(9):1656–1667. https://doi.org/10.1016/j.ejso.2017.04.015
Liberale G et al (2018) Indocyanine green fluorescence imaging for sentinel lymph node detection in colorectal cancer: a systematic review. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 44(9):1301–1306. https://doi.org/10.1016/j.ejso.2018.05.034
Mizrahi I, Wexner SD (2017) Clinical role of fluorescence imaging in colorectal surgery—a review. Expert Rev Med Devices 14(1):75–82. https://doi.org/10.1080/17434440.2017.1265444
Nachiappan S, Askari A, Currie A, Kennedy RH, Faiz O (2014) Intraoperative assessment of colorectal anastomotic integrity: a systematic review. Surg Endosc 28(9):2513–2530. https://doi.org/10.1007/s00464-014-3520-z
Tejedor P, Sagias F, Khan JS (2020) The use of enhanced technologies in robotic surgery and its impact on outcomes in rectal cancer: a systematic review. Surg Innov 27(4):384–391. https://doi.org/10.1177/1553350620928277
Koyanagi K et al (2022) Indocyanine green fluorescence imaging for evaluating blood flow in the reconstructed conduit after esophageal cancer surgery. Surg Today 52(3):369–376. https://doi.org/10.1007/s00595-021-02296-4
Van Daele E et al (2019) Near-infrared fluorescence guided esophageal reconstructive surgery: a systematic review. World J Gastrointest Oncol 11(3):250–263. https://doi.org/10.4251/wjgo.v11.i3.250
Can MF, Yagci G, Cetiner S (2013) Systematic review of studies investigating sentinel node navigation surgery and lymphatic mapping for gastric cancer. J Laparoendosc Adv Surg Tech A 23(8):651–662. https://doi.org/10.1089/lap.2012.0311
Felli E et al (2021) Laparoscopic anatomical liver resection for malignancies using positive or negative staining technique with intraoperative indocyanine green-fluorescence imaging. HPB 23(11):1647–1655. https://doi.org/10.1016/j.hpb.2021.05.006
Potharazu AV, Gangemi A (2023) Indocyanine green (ICG) fluorescence in robotic hepatobiliary surgery: a systematic review. Int. J. Med. Robot. Comput. Assist. Surg. 19(1):e2485. https://doi.org/10.1002/rcs.2485
Tomassini F, Giglio MC, De Simone G, Montalti R, Troisi RI (2020) Hepatic function assessment to predict post-hepatectomy liver failure: what can we trust? A systematic review. Updat Surg 72(4):925–938. https://doi.org/10.1007/s13304-020-00859-7
Wakabayashi T et al (2022) Indocyanine green fluorescence navigation in liver surgery: a systematic review on dose and timing of administration. Ann Surg 275(6):1025–1034. https://doi.org/10.1097/SLA.0000000000005406
Al-Taher M et al (2018) Intraoperative enhanced imaging for detection of endometriosis: a systematic review of the literature. Eur J Obstet Gynecol Reprod Biol 224:108–116. https://doi.org/10.1016/j.ejogrb.2018.03.020
Boussedra S et al (2022) Fluorescence guided surgery to improve peritoneal cytoreduction in epithelial ovarian cancer: a systematic review of available data. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 48(6):1217–1223. https://doi.org/10.1016/j.ejso.2022.02.022
Dell’Orto F, Laven P, Delle Marchette M, Lambrechts S, Kruitwagen R, Buda A (2019) Feasibility of sentinel lymph node mapping of the ovary: a systematic review. Int J Gynecol Cancer 29(7):1209–1215. https://doi.org/10.1136/ijgc-2019-000606
Ianieri MM et al (2021) Indocyanine green in the surgical management of endometriosis: a systematic review. Acta Obstet Gynecol Scand 100(2):189–199. https://doi.org/10.1111/aogs.13971
Koual M, Benoit L, Nguyen-Xuan H-T, Bentivegna E, Azaïs H, Bats A-S (2021) Diagnostic value of indocyanine green fluorescence guided sentinel lymph node biopsy in vulvar cancer: a systematic review. Gynecol Oncol 161(2):436–441. https://doi.org/10.1016/j.ygyno.2021.01.031
Raffone A et al (2022) The use of near infra-red radiation imaging after injection of indocyanine green (NIR-ICG) during laparoscopic treatment of benign gynecologic conditions: towards minimalized surgery: a systematic review of literature. Med. Kaunas Lith. 58(6):792. https://doi.org/10.3390/medicina58060792
Rocha A, Domínguez AM, Lécuru F, Bourdel N (2016) Indocyanine green and infrared fluorescence in detection of sentinel lymph nodes in endometrial and cervical cancer staging—a systematic review. Eur J Obstet Gynecol Reprod Biol 206:213–219. https://doi.org/10.1016/j.ejogrb.2016.09.027
Wang X, Zhang Y, Yang H, Xu Y (2022) Maternal-fetal transfer of indocyanine green: a systematic review. J Matern Fetal Neonatal Med 35(25):8181–8185. https://doi.org/10.1080/14767058.2021.1966410
Zapardiel I et al (2021) Utility of intraoperative fluorescence imaging in gynecologic surgery: systematic review and consensus statement. Ann Surg Oncol 28(6):3266–3278. https://doi.org/10.1245/s10434-020-09222-x
Alghoul H, Farajat FA, Alser O, Snyr AR, Harmon CM, Novotny NM (2022) Intraoperative uses of near-infrared fluorescence spectroscopy in pediatric surgery: a systematic review. J Pediatr Surg 57(6):1137–1144. https://doi.org/10.1016/j.jpedsurg.2022.01.039
Breuking EA, van Varsseveld OC, Harms M, Tytgat SHAJ, Hulscher JBF, Ruiterkamp J (2023) Safety and feasibility of indocyanine green fluorescence angiography in pediatric gastrointestinal surgery: a systematic review. J Pediatr Surg 58(8):1534–1542. https://doi.org/10.1016/j.jpedsurg.2022.10.045
Le-Nguyen A, O’Neill Trudeau M, Dodin P, Keezer MR, Faure C, Piché N (2021) The use of indocyanine green fluorescence angiography in pediatric surgery: a systematic review and narrative analysis. Front Pediatr 9:736242. https://doi.org/10.3389/fped.2021.736242
Paraboschi I, De Coppi P, Stoyanov D, Anderson J, Giuliani S (2021) Fluorescence imaging in pediatric surgery: state-of-the-art and future perspectives. J Pediatr Surg 56(4):655–662. https://doi.org/10.1016/j.jpedsurg.2020.08.004
Baiocchi GL, Gheza F, Molfino S, Arru L, Vaira M, Giacopuzzi S (2020) Indocyanine green fluorescence-guided intraoperative detection of peritoneal carcinomatosis: systematic review. BMC Surg 20(1):158. https://doi.org/10.1186/s12893-020-00821-9
Sposito C et al (2022) Indocyanine green fluorescence-guided surgery for gastrointestinal tumors: a systematic review. Ann Surg Open 3(3):e190. https://doi.org/10.1097/AS9.0000000000000190
Autorino R et al (2014) Current applications of near-infrared fluorescence imaging in robotic urologic surgery: a systematic review and critical analysis of the literature. Urology 84(4):751–759. https://doi.org/10.1016/j.urology.2014.05.059
Cacciamani GE et al (2020) Best practices in near-infrared fluorescence imaging with indocyanine green (NIRF/ICG)-guided robotic urologic surgery: a systematic review-based expert consensus. World J Urol 38(4):883–896. https://doi.org/10.1007/s00345-019-02870-z
Rodler S et al (2023) A systematic review of new imaging technologies for robotic prostatectomy: from molecular imaging to augmented reality. J Clin Med 12(16):5425. https://doi.org/10.3390/jcm12165425
Al-Taher M et al (2022) Near infrared fluorescence imaging of the urethra: a systematic review of the literature. Minim Invasive Ther Allied Technol 31(3):342–349. https://doi.org/10.1080/13645706.2020.1826974
Degett TH, Andersen HS, Gögenur I (2016) Indocyanine green fluorescence angiography for intraoperative assessment of gastrointestinal anastomotic perfusion: a systematic review of clinical trials. Langenbecks Arch Surg 401(6):767–775. https://doi.org/10.1007/s00423-016-1400-9
Schols RM, Connell NJ, Stassen LPS (2015) Near-infrared fluorescence imaging for real-time intraoperative anatomical guidance in minimally invasive surgery: a systematic review of the literature. World J Surg 39(5):1069–1079. https://doi.org/10.1007/s00268-014-2911-6
Slooter MD, Janssen A, Bemelman WA, Tanis PJ, Hompes R (2019) Currently available and experimental dyes for intraoperative near-infrared fluorescence imaging of the ureters: a systematic review. Tech Coloproctol 23(4):305–313. https://doi.org/10.1007/s10151-019-01973-4
Slooter MD et al (2021) Defining indocyanine green fluorescence to assess anastomotic perfusion during gastrointestinal surgery: systematic review. BJS Open 5(2):zraa074. https://doi.org/10.1093/bjsopen/zraa074
Dip F, LoMenzo E, White KP, Rosenthal RJ (2021) Does near-infrared fluorescent cholangiography with indocyanine green reduce bile duct injuries and conversions to open surgery during laparoscopic or robotic cholecystectomy? A meta-analysis. Surgery 169(4):859–867. https://doi.org/10.1016/j.surg.2020.12.008
Lim SH, Tan HTA, Shelat VG (2021) Comparison of indocyanine green dye fluorescent cholangiography with intra-operative cholangiography in laparoscopic cholecystectomy: a meta-analysis. Surg Endosc 35(4):1511–1520. https://doi.org/10.1007/s00464-020-08164-5
Liu Y et al (2020) A meta-analysis of indocyanine green fluorescence image-guided laparoscopic cholecystectomy for benign gallbladder disease. Photodiagnosis Photodyn Ther 32:101948. https://doi.org/10.1016/j.pdpdt.2020.101948
Vlek SL et al (2017) Biliary tract visualization using near-infrared imaging with indocyanine green during laparoscopic cholecystectomy: results of a systematic review. Surg Endosc 31(7):2731–2742. https://doi.org/10.1007/s00464-016-5318-7
Ankersmit M, Bonjer HJ, Hannink G, Schoonmade LJ, van der Pas MHGM, Meijerink WJHJ (2019) Near-infrared fluorescence imaging for sentinel lymph node identification in colon cancer: a prospective single-center study and systematic review with meta-analysis. Tech Coloproctol 23(12):1113–1126. https://doi.org/10.1007/s10151-019-02107-6
Arezzo A et al (2020) Intraoperative use of fluorescence with indocyanine green reduces anastomotic leak rates in rectal cancer surgery: an individual participant data analysis. Surg Endosc 34(10):4281–4290. https://doi.org/10.1007/s00464-020-07735-w
Blanco-Colino R, Espin-Basany E (2018) Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: a systematic review and meta-analysis. Tech Coloproctol 22(1):15–23. https://doi.org/10.1007/s10151-017-1731-8
Chan DKH, Lee SKF, Ang JJ (2020) Indocyanine green fluorescence angiography decreases the risk of colorectal anastomotic leakage: systematic review and meta-analysis. Surgery 168(6):1128–1137. https://doi.org/10.1016/j.surg.2020.08.024
Deng J et al (2022) Meta analysis of indocyanine green fluorescence in patients undergoing laparoscopic colorectal cancer surgery. Front Oncol 12:1010122. https://doi.org/10.3389/fonc.2022.1010122
Emile SH et al (2017) Sensitivity and specificity of indocyanine green near-infrared fluorescence imaging in detection of metastatic lymph nodes in colorectal cancer: systematic review and meta-analysis. J Surg Oncol 116(6):730–740. https://doi.org/10.1002/jso.24701
Emile SH, Khan SM, Wexner SD (2022) Impact of change in the surgical plan based on indocyanine green fluorescence angiography on the rates of colorectal anastomotic leak: a systematic review and meta-analysis. Surg Endosc 36(4):2245–2257. https://doi.org/10.1007/s00464-021-08973-2
Kryzauskas M et al (2020) Intraoperative testing of colorectal anastomosis and the incidence of anastomotic leak: a meta-analysis. Medicine (Baltimore) 99(47):e23135. https://doi.org/10.1097/MD.0000000000023135
Li Z et al (2021) Meta-analysis on the efficacy of indocyanine green fluorescence angiography for reduction of anastomotic leakage after rectal cancer surgery. Am Surg 87(12):1910–1919. https://doi.org/10.1177/0003134820982848
Lin J, Zheng B, Lin S, Chen Z, Chen S (2021) The efficacy of intraoperative ICG fluorescence angiography on anastomotic leak after resection for colorectal cancer: a meta-analysis. Int J Colorectal Dis 36(1):27–39. https://doi.org/10.1007/s00384-020-03729-1
Liu D, Liang L, Liu L, Zhu Z (2021) Does intraoperative indocyanine green fluorescence angiography decrease the incidence of anastomotic leakage in colorectal surgery? A systematic review and meta-analysis. Int J Colorectal Dis 36(1):57–66. https://doi.org/10.1007/s00384-020-03741-5
Lucas K et al (2023) Lymphatic mapping in colon cancer depending on injection time and tracing agent: a systematic review and meta-analysis of prospective designed studies. Cancers 15(12):3196. https://doi.org/10.3390/cancers15123196
Mok HT et al (2020) Indocyanine green fluorescent imaging on anastomotic leakage in colectomies: a network meta-analysis and systematic review. Int J Colorectal Dis 35(12):2365–2369. https://doi.org/10.1007/s00384-020-03723-7
Pang H-Y et al (2021) Indocyanine green fluorescence angiography prevents anastomotic leakage in rectal cancer surgery: a systematic review and meta-analysis. Langenbecks Arch Surg 406(2):261–271. https://doi.org/10.1007/s00423-020-02077-6
Qiao L (2020) Sentinel lymph node mapping for metastasis detection in colorectal cancer: a systematic review and meta-analysis. Rev Espanola Enfermedades Dig 112(9):722–730. https://doi.org/10.17235/reed.2020.6767/2019
Rausa E et al (2019) A standardized use of intraoperative anastomotic testing in colorectal surgery in the new millennium: is technology taking over? A systematic review and network meta-analysis. Tech Coloproctol 23(7):625–631. https://doi.org/10.1007/s10151-019-02034-6
Renna MS et al (2023) Intraoperative bowel perfusion assessment methods and their effects on anastomotic leak rates: meta-analysis. Br J Surg 110(9):1131–1142. https://doi.org/10.1093/bjs/znad154
Safiejko K et al (2022) Safety and efficacy of indocyanine green in colorectal cancer surgery: a systematic review and meta-analysis of 11,047 patients. Cancers 14(4):1036. https://doi.org/10.3390/cancers14041036
Shen R, Zhang Y, Wang T (2018) Indocyanine green fluorescence angiography and the incidence of anastomotic leak after colorectal resection for colorectal cancer: a meta-analysis. Dis Colon Rectum 61(10):1228–1234. https://doi.org/10.1097/DCR.0000000000001123
Shen Y, Yang T, Yang J, Meng W, Wang Z (2020) Intraoperative indocyanine green fluorescence angiography to prevent anastomotic leak after low anterior resection for rectal cancer: a meta-analysis. ANZ J Surg 90(11):2193–2200. https://doi.org/10.1111/ans.15809
Song M et al (2021) Assessment of intraoperative use of indocyanine green fluorescence imaging on the incidence of anastomotic leakage after rectal cancer surgery: a PRISMA-compliant systematic review and meta-analysis. Tech Coloproctol 25(1):49–58. https://doi.org/10.1007/s10151-020-02335-1
Tang G, Du D, Tao J, Wei Z (2022) Effect of indocyanine green fluorescence angiography on anastomotic leakage in patients undergoing colorectal surgery: a meta-analysis of randomized controlled trials and propensity-score-matched studies. Front Surg 9:815753. https://doi.org/10.3389/fsurg.2022.815753
Trastulli S, Munzi G, Desiderio J, Cirocchi R, Rossi M, Parisi A (2021) Indocyanine green fluorescence angiography versus standard intraoperative methods for prevention of anastomotic leak in colorectal surgery: meta-analysis. Br J Surg 108(4):359–372. https://doi.org/10.1093/bjs/znaa139
Villegas-Tovar E et al (2020) Performance of Indocyanine green for sentinel lymph node mapping and lymph node metastasis in colorectal cancer: a diagnostic test accuracy meta-analysis. Surg Endosc 34(3):1035–1047. https://doi.org/10.1007/s00464-019-07274-z
Xia S, Wu W, Luo L, Ma L, Yu L, Li Y (2023) Indocyanine green fluorescence angiography decreases the risk of anastomotic leakage after rectal cancer surgery: a systematic review and meta-analysis. Front Med 10:1157389. https://doi.org/10.3389/fmed.2023.1157389
Zhang W, Che X (2021) Effect of indocyanine green fluorescence angiography on preventing anastomotic leakage after colorectal surgery: a meta-analysis. Surg Today 51(9):1415–1428. https://doi.org/10.1007/s00595-020-02195-0
Casas MA, Angeramo CA, Bras Harriott C, Dreifuss NH, Schlottmann F (2022) Indocyanine green (ICG) fluorescence imaging for prevention of anastomotic leak in totally minimally invasive Ivor Lewis esophagectomy: a systematic review and meta-analysis. Dis Esophagus 35(4):doab056. https://doi.org/10.1093/dote/doab056
Hong Z-N, Huang L, Zhang W, Kang M (2022) Indocyanine green fluorescence using in conduit reconstruction for patients with esophageal cancer to improve short-term clinical outcome: a meta-analysis. Front Oncol 12:847510. https://doi.org/10.3389/fonc.2022.847510
Jimenez-Lillo J et al (2021) Performance of indocyanine-green imaging for sentinel lymph node mapping and lymph node metastasis in esophageal cancer: systematic review and meta-analysis. Ann Surg Oncol 28(9):4869–4877. https://doi.org/10.1245/s10434-021-09617-4
Ladak F et al (2019) Indocyanine green for the prevention of anastomotic leaks following esophagectomy: a meta-analysis. Surg Endosc 33(2):384–394. https://doi.org/10.1007/s00464-018-6503-7
Slooter MD, Eshuis WJ, Cuesta MA, Gisbertz SS, van Berge Henegouwen MI (2019) Fluorescent imaging using indocyanine green during esophagectomy to prevent surgical morbidity: a systematic review and meta-analysis. J Thorac Dis 11(Suppl 5):S755–S765. https://doi.org/10.21037/jtd.2019.01.30
Deng C et al (2022) Safety and efficacy of indocyanine green near-infrared fluorescent imaging-guided lymph nodes dissection during radical gastrectomy for gastric cancer: a systematic review and meta-analysis. Front Oncol 12:917541. https://doi.org/10.3389/fonc.2022.917541
Dong B, Zhang A, Zhang Y, Ye W, Liao L, Li Z (2022) Efficacy of indocyanine green fluorescence imaging-guided lymphadenectomy in radical gastrectomy for gastric cancer: a systematic review and meta-analysis. Front Oncol 12:998159. https://doi.org/10.3389/fonc.2022.998159
He M, Jiang Z, Wang C, Hao Z, An J, Shen J (2018) Diagnostic value of near-infrared or fluorescent indocyanine green guided sentinel lymph node mapping in gastric cancer: a systematic review and meta-analysis. J Surg Oncol 118(8):1243–1256. https://doi.org/10.1002/jso.25285
Huang L, Wei T, Chen J, Zhou D (2017) Feasibility and diagnostic performance of dual-tracer-guided sentinel lymph node biopsy in cT1-2N0M0 gastric cancer: a systematic review and meta-analysis of diagnostic studies. World J Surg Oncol 15(1):103. https://doi.org/10.1186/s12957-017-1159-7
Pang H-Y et al (2022) Assessment of indocyanine green fluorescence lymphography on lymphadenectomy during minimally invasive gastric cancer surgery: a systematic review and meta-analysis. Surg Endosc 36(3):1726–1738. https://doi.org/10.1007/s00464-021-08830-2
Skubleny D et al (2018) Diagnostic evaluation of sentinel lymph node biopsy using indocyanine green and infrared or fluorescent imaging in gastric cancer: a systematic review and meta-analysis. Surg Endosc 32(6):2620–2631. https://doi.org/10.1007/s00464-018-6100-9
Zhao J et al (2022) Efficacy and safety of indocyanine green tracer-guided lymph node dissection in minimally invasive radical gastrectomy for gastric cancer: a systematic review and meta-analysis. Front Oncol 12:884011. https://doi.org/10.3389/fonc.2022.884011
Chen H et al (2022) Application effect of icg fluorescence real-time imaging technology in laparoscopic hepatectomy. Front Oncol 12:819960. https://doi.org/10.3389/fonc.2022.819960
Granieri S et al (2022) Preoperative Indocyanine Green (ICG) clearance test: can we really trust it to predict post hepatectomy liver failure? A systematic review of the literature and meta-analysis of diagnostic test accuracy. Photodiagnosis Photodyn Ther. https://doi.org/10.1016/j.pdpdt.2022.103170
Hu Y, Fu T, Zhang Z, Hua L, Zhao Q, Zhang W (2021) Does application of indocyanine green fluorescence imaging enhance clinical outcomes in liver resection? A meta-analysis. Photodiagnosis Photodyn Ther 36:102554. https://doi.org/10.1016/j.pdpdt.2021.102554
Liu Y, Wang Q, Du B, Wang XZ, Xue Q, Gao WF (2021) Meta-analysis of indocyanine green fluorescence imaging-guided laparoscopic hepatectomy. Photodiagnosis Photodyn Ther 35:102354. https://doi.org/10.1016/j.pdpdt.2021.102354
Purich K et al (2020) Intraoperative fluorescence imaging with indocyanine green in hepatic resection for malignancy: a systematic review and meta-analysis of diagnostic test accuracy studies. Surg Endosc 34(7):2891–2903. https://doi.org/10.1007/s00464-020-07543-2
Qi C et al (2019) Effectiveness and safety of indocyanine green fluorescence imaging-guided hepatectomy for liver tumors: a systematic review and first meta-analysis. Photodiagnosis Photodyn Ther 28:346–353. https://doi.org/10.1016/j.pdpdt.2019.10.007
Rompianesi G et al (2022) Systematic review, meta-analysis and single-centre experience of the diagnostic accuracy of intraoperative near-infrared indocyanine green-fluorescence in detecting pancreatic tumours. HPB 24(11):1823–1831. https://doi.org/10.1016/j.hpb.2022.05.004
Wang H-Q, Yang J, Yang J-Y, Yan L-N (2013) Bile leakage test in liver resection: a systematic review and meta-analysis. World J Gastroenterol 19(45):8420–8426. https://doi.org/10.3748/wjg.v19.i45.8420
Wang J, Xu Y, Zhang Y, Tian H (2023) Safety and effectiveness of fluorescence laparoscopy in precise hepatectomy: a meta-analysis. Photodiagnosis Photodyn Ther 42:103599. https://doi.org/10.1016/j.pdpdt.2023.103599
Xu C, Cui X, Jia Z, Shen X, Che J (2023) A meta-analysis of short-term and long-term effects of indocyanine green fluorescence imaging in hepatectomy for liver cancer. Photodiagnosis Photodyn Ther 42:103497. https://doi.org/10.1016/j.pdpdt.2023.103497
Xue Q, Wu J, Lei Z, Wang Q, Gao W, Fu J (2022) Application value of fluorescence visualization-assisted technology in the resection of liver cancer: a systematic review and meta-analysis. Photodiagnosis Photodyn Ther 39:102940. https://doi.org/10.1016/j.pdpdt.2022.102940
Zhu G et al (2023) Application of indocyanine green-mediated fluorescence molecular imaging technology in liver tumors resection: a systematic review and meta-analysis. Front Oncol 13:1167536. https://doi.org/10.3389/fonc.2023.1167536
Baeten IGT et al (2022) Indocyanine green versus technetium-99m with blue dye for sentinel lymph node detection in early-stage cervical cancer: a systematic review and meta-analysis. Cancer Rep Hoboken NJ 5(1):e1401. https://doi.org/10.1002/cnr2.1401
Bodurtha Smith AJ, Fader AN, Tanner EJ (2017) Sentinel lymph node assessment in endometrial cancer: a systematic review and meta-analysis. Am J Obstet Gynecol 216(5):459–476. https://doi.org/10.1016/j.ajog.2016.11.1033
Burg LC et al (2022) The added value of SLN mapping with indocyanine green in low- and intermediate-risk endometrial cancer management: a systematic review and meta-analysis. J Gynecol Oncol 33(5):e66. https://doi.org/10.3802/jgo.2022.33.e66
Chiyoda T et al (2022) Sentinel node navigation surgery in cervical cancer: a systematic review and metaanalysis. Int J Clin Oncol 27(8):1247–1255. https://doi.org/10.1007/s10147-022-02178-w
Crivellaro C et al (2018) Sentinel node biopsy in endometrial cancer: an update. Clin Transl Imaging 6(2):91–100. https://doi.org/10.1007/s40336-018-0268-9
Di Donna MC et al (2023) Detection of sentinel lymph node in vulvar cancer using 99mTc-labeled colloid lymphoscintigraphy, blue dye, and indocyanine-green fluorescence: a meta-analysis of studies published in 2010–2020. Arch Gynecol Obstet 307(6):1677–1686. https://doi.org/10.1007/s00404-022-06605-1
Ji Q, Wang X, Jiang J, Chen L (2020) Sentinel lymph node mapping in high-risk endometrial cancer: a systematic review and meta-analysis. Gland Surg 9(6):2091–2105. https://doi.org/10.21037/gs-20-807
Lin H, Ding Z, Kota VG, Zhang X, Zhou J (2017) Sentinel lymph node mapping in endometrial cancer: a systematic review and meta-analysis. Oncotarget 8(28):46601–46610. https://doi.org/10.18632/oncotarget.16662
Maheux-Lacroix S, Belanger M, Pinard L, Lemyre M, Laberge P, Boutin A (2020) Diagnostic accuracy of intraoperative tools for detecting endometriosis: a systematic review and meta-analysis. J Minim Invasive Gynecol 27(2):433–440. https://doi.org/10.1016/j.jmig.2019.11.010
Marchocki Z et al (2021) Sentinel lymph node biopsy in high-grade endometrial cancer: a systematic review and meta-analysis of performance characteristics. Am J Obstet Gynecol 225(4):367.e1-367.e39. https://doi.org/10.1016/j.ajog.2021.05.034
Nagar H, Wietek N, Goodall RJ, Hughes W, Schmidt-Hansen M, Morrison J (2021) Sentinel node biopsy for diagnosis of lymph node involvement in endometrial cancer. Cochrane Database Syst Rev 6(6):CD013021. https://doi.org/10.1002/14651858.CD013021.pub2
Ruscito I et al (2016) Sentinel node mapping in cervical and endometrial cancer: indocyanine green versus other conventional dyes-a meta-analysis. Ann Surg Oncol 23(11):3749–3756. https://doi.org/10.1245/s10434-016-5236-x
Ulain Q et al (2018) Indocyanine green can stand alone in detecting sentinel lymph nodes in cervical cancer. J Int Med Res 46(12):4885–4897. https://doi.org/10.1177/0300060518803041
Wang L, Liu S, Xu T, Yuan L, Yang X (2021) Sentinel lymph node mapping in early-stage cervical cancer: meta-analysis. Medicine (Baltimore) 100(34):e27035. https://doi.org/10.1097/MD.0000000000027035
Zhang X, Bao B, Wang S, Yi M, Jiang L, Fang X (2021) Sentinel lymph node biopsy in early stage cervical cancer: a meta-analysis. Cancer Med 10(8):2590–2600. https://doi.org/10.1002/cam4.3645
Wu Y, Jing J, Wang J, Xu B, Du M, Chen M (2019) Robotic-assisted sentinel lymph node mapping with indocyanine green in pelvic malignancies: a systematic review and meta-analysis. Front Oncol 9:585. https://doi.org/10.3389/fonc.2019.00585
Xiong L et al (2014) Indocyanine green fluorescence-guided sentinel node biopsy: a meta-analysis on detection rate and diagnostic performance. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 40(7):843–849. https://doi.org/10.1016/j.ejso.2014.02.228
Aoun F, Albisinni S, Zanaty M, Hassan T, Janetschek G, van Velthoven R (2018) Indocyanine green fluorescence-guided sentinel lymph node identification in urologic cancers: a systematic review and meta-analysis. Miverva Urol E Nefrol Ital J Urol Nephrol 70(4):361–369. https://doi.org/10.23736/S0393-2249.17.02932-0
Urabe F et al (2021) Performance of indocyanine green fluorescence for detecting lymph node metastasis in prostate cancer: a systematic review and meta-analysis. Clin Genitourin Cancer 19(5):466.e1-466.e9. https://doi.org/10.1016/j.clgc.2021.03.013
Veccia A et al (2020) Near-infrared fluorescence imaging with indocyanine green in robot-assisted partial nephrectomy: pooled analysis of comparative studies. Eur Urol Focus 6(3):505–512. https://doi.org/10.1016/j.euf.2019.03.005
Zhang R, Zhang Y, Dong S, Pang K, Yang X, Wei X (2023) Performance of indocyanine green in sentinel lymph node mapping and lymph node metastasis in penile cancer: systematic review, meta-analysis, and single-center experience. World J Urol 41(9):2319–2326. https://doi.org/10.1007/s00345-023-04485-x
Ortega CB, Guerron AD, Yoo JS (2018) The use of fluorescence angiography during laparoscopic sleeve gastrectomy. JSLS 22(2):e2018.00005. https://doi.org/10.4293/JSLS.2018.00005
Mangano A et al (2022) Role of Indocyanine Green (ICG)-enhanced fluorescence in primary and revisional bariatric surgery: narrative overview of selected literature and intraoperative surgical videos. Surg Technol Int 40:79–84. https://doi.org/10.52198/22.STI.40.GS1517
Mongelli F et al (2022) Reoperative bariatric surgery after primary laparoscopic gastric plication for morbid obesity: a systematic review and meta-analysis. Langenbecks Arch Surg 407(5):1839–1850. https://doi.org/10.1007/s00423-022-02485-w
Gupta V, Jain G (2019) Safe laparoscopic cholecystectomy: adoption of universal culture of safety in cholecystectomy. World J Gastrointest Surg 11(2):62–84. https://doi.org/10.4240/wjgs.v11.i2.62
You J et al (2022) Intrathoracic versus cervical anastomosis in esophagectomy for esophageal cancer: a meta-analysis of randomized controlled trials. Surgery 172(2):575–583. https://doi.org/10.1016/j.surg.2022.03.006
Verstegen MHP et al (2019) Management of intrathoracic and cervical anastomotic leakage after esophagectomy for esophageal cancer: a systematic review. World J Emerg Surg 14(1):17. https://doi.org/10.1186/s13017-019-0235-4
Wang Y, Zhu L, Xia W, Wang F (2018) Anatomy of lymphatic drainage of the esophagus and lymph node metastasis of thoracic esophageal cancer. Cancer Manag Res 10:6295. https://doi.org/10.2147/CMAR.S182436
Prenzel KL et al (2010) Prognostic relevance of skip metastases in esophageal cancer. Ann Thorac Surg 90(5):1662–1667. https://doi.org/10.1016/j.athoracsur.2010.07.008
Ishizawa T et al (2009) Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 115(11):2491–2504. https://doi.org/10.1002/cncr.24291
Ishizawa T, Zuker NB, Kokudo N, Gayet B (2012) Positive and negative staining of hepatic segments by use of fluorescent imaging techniques during laparoscopic hepatectomy. Arch Surg 147(4):393–394. https://doi.org/10.1001/archsurg.2012.59
Dip F et al (2022) Consensus conference statement on the general use of near-infrared fluorescence imaging and indocyanine green guided surgery: results of a modified Delphi study. Ann Surg 275(4):685. https://doi.org/10.1097/SLA.0000000000004412
Pollmann L, Juratli M, Roushansarai N, Pascher A, Hölzen JP (2023) Quantification of indocyanine green fluorescence imaging in general, visceral and transplant surgery. J Clin Med 12(10):3550. https://doi.org/10.3390/jcm12103550
Spota A et al (2021) Fluorescence-based bowel anastomosis perfusion evaluation: results from the IHU-IRCAD-EAES EURO-FIGS registry. Surg Endosc 35(12):7142–7153. https://doi.org/10.1007/s00464-020-08234-8
Zhou S-N et al (2023) Feasibility of machine learning-based modeling and prediction using multiple centers data to assess intrahepatic cholangiocarcinoma outcomes. Ann Med 55(1):215–223. https://doi.org/10.1080/07853890.2022.2160008
Ishizawa T et al (2021) Assessing the development status of intraoperative fluorescence imaging for perfusion assessments, using the IDEAL framework. BMJ Surg Interv Health Technol 3(1):e000088. https://doi.org/10.1136/bmjsit-2021-000088
Shea BJ et al (2007) Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 7(1):10. https://doi.org/10.1186/1471-2288-7-10
Faggion CM (2015) Critical appraisal of AMSTAR: challenges, limitations, and potential solutions from the perspective of an assessor. BMC Med Res Methodol 15(1):63. https://doi.org/10.1186/s12874-015-0062-6
Acknowledgements
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Athanasios G. Pantelis, Nikolaos Machairiotis, Sofoklis Stavros, Stewart Disu, and Petros Drakakis has no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
464_2023_10546_MOESM3_ESM.tif
Supplementary Figure 2. Distribution of the included systematic reviews (SR) and meta-analyses (MA) by scientific discipline / (sub)specialty
464_2023_10546_MOESM4_ESM.tif
Supplementary Figure 3. Distribution of the included meta-analyses by (sub)specialty and by overall quality score according to the AMSTAR 2 instrument
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pantelis, A.G., Machairiotis, N., Stavros, S. et al. Current applications of indocyanine green (ICG) in abdominal, gynecologic and urologic surgery: a meta-review and quality analysis with use of the AMSTAR 2 instrument. Surg Endosc 38, 511–528 (2024). https://doi.org/10.1007/s00464-023-10546-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10546-4