Abstract
Background
Vonoprazan is a new acid-suppressing drug that received FDA approval in 2022. It reversibly inhibits gastric acid secretion by competing with the potassium ions on the luminal surface of the parietal cells (potassium-competitive acid blockers or P-CABs). Vonoprazan has been on the market for a short time and there are many clinical trials to support its clinical application. However, medical experience and comprehensive clinical data is still limited, especially on how and if, gastric histology is altered due to therapy.
Methods
A 12-week experiment trial with 30 Wistar rats was to assess the presence of gastrointestinal morphologic abnormalities upon administration of omeprazole and vonoprazan. At six weeks of age, rats were randomly assigned to one of 5 groups: (1) saline as negative control group, (2) oral omeprazole (40 mg/kg), as positive control group, (3) oral omeprazole (40 mg/kg) for 4 weeks, proceeded by 8 weeks off omeprazole, (4) oral vonoprazan (4 mg/kg), as positive control group, and (5) oral vonoprazan (4 mg/kg) for 4 weeks, proceeded by 8 weeks off vonoprazan.
Results
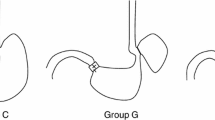
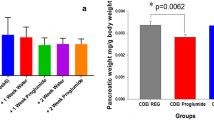
We identified non-inflammatory alterations characterized by parietal (oxyntic) cell loss and chief (zymogen) cell hyperplasia and replacement by pancreatic acinar cell metaplasia (PACM). No significant abnormalities were identified in any other tissues in the hepatobiliary and gastrointestinal tracts.
Conclusion
PACM has been reported in gastric mucosa, at the esophagogastric junction, at the distal esophagus, and in Barrett esophagus. However, the pathogenesis of this entity is still unclear. Whereas some authors have suggested that PACM is an acquired process others have raised the possibility of PACM being congenital in nature. Our results suggest that the duration of vonoprazan administration at a dose of 4 mg/kg plays an important role in the development of PACM.


Similar content being viewed by others
References
Dent J et al (2012) Systematic review: patterns of reflux-induced symptoms and esophageal endoscopic findings in large-scale surveys. Clin Gastroenterol Hepatol 10(8):863-873.e3
Peery AF et al (2019) Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology 156(1):254-272.e11
Mittal R, Vaezi MF (2020) Esophageal motility disorders and gastroesophageal reflux disease. N Engl J Med 383(20):1961–1972
Vakil N (2012) Prescribing proton pump inhibitors: is it time to pause and rethink? Drugs 72(4):437–445
Heidelbaugh JJ et al (2012) Overutilization of proton-pump inhibitors: what the clinician needs to know. Ther Adv Gastroenterol 5(4):219–232
Savarino V et al (2017) The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med 37:19–24
Yadlapati R, Kahrilas PJ (2017) When is proton pump inhibitor use appropriate? BMC Med 15(1):36
Schoenfeld AJ, Grady D (2016) Adverse effects associated with proton pump inhibitors. JAMA Intern Med 176(2):172–174
Jenkins H et al (2015) Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther 41(7):636–648
Echizen H (2016) The first-in-class potassium-competitive acid blocker, vonoprazan fumarate: pharmacokinetic and pharmacodynamic considerations. Clin Pharmacokinet 55(4):409–418
Chen F et al (2020) In Vitro and in vivo rat model assessments of the effects of vonoprazan on the pharmacokinetics of venlafaxine. Drug Des Dev Ther 14:4815–4824
Garnock-Jones KP (2015) Vonoprazan: first global approval. Drugs 75(4):439–443
Kong WM et al (2020) Physiologically based pharmacokinetic-pharmacodynamic modeling for prediction of vonoprazan pharmacokinetics and its inhibition on gastric acid secretion following intravenous/oral administration to rats, dogs and humans. Acta Pharmacol Sin 41(6):852–865
Hori Y et al (2011) A study comparing the antisecretory effect of TAK-438, a novel potassium-competitive acid blocker, with lansoprazole in animals. J Pharmacol Exp Ther 337(3):797–804
Qiao Y et al (2017) Study on pharmacokinetics and bioequivalence of Vonoprazan pyroglutamate in rats by liquid chromatography with tandem mass spectrometry. J Chromatogr B 1059:56–65
Sakurai Y et al (2015) Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK-438 (Vonoprazan) doses in healthy male Japanese/non-Japanese subjects. Clin Transl Gastroenterol 6(6):e94
Suzuki T et al (2018) Comparison of effect of an increased dosage of vonoprazan versus vonoprazan plus lafutidine on gastric acid inhibition and serum gastrin. Eur J Clin Pharmacol 74(1):45–52
Sakurai Y et al (2016) Pharmacokinetics and safety of triple therapy with vonoprazan, amoxicillin, and clarithromycin or metronidazole: a phase 1, open-label, randomized, crossover study. Adv Ther 33(9):1519–1535
Sakurai Y et al (2015) Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects–a randomised open-label cross-over study. Aliment Pharmacol Ther 42(6):719–730
Jenkins H, Jenkins R, Patat A (2017) Effect of multiple oral doses of the potent CYP3A4 inhibitor clarithromycin on the pharmacokinetics of a single oral dose of vonoprazan: a phase I, open-label, sequential design study. Clin Drug Investig 37(3):311–316
Suckow M et al (2019) The laboratory rat. Elsevier, Amsterdam
Schneider NI et al (2013) Pancreatic acinar cells: a normal finding at the gastroesophageal junction? Data from a prospective Central European multicenter study. Virchows Arch 463(5):643–650
Wang HH et al (1996) Prevalence and significance of pancreatic acinar metaplasia at the gastroesophageal junction. Am J Surg Pathol 20(12):1507–1510
Al Salihi S et al (2019) Pancreatic acinar metaplasia in distal esophageal biopsies is associated with chronic nonsteroidal anti-inflammatory drug use. Arch Pathol Lab Med 143(4):510–512
Glickman JN et al (2002) Morphology of the cardia and significance of carditis in pediatric patients. Am J Surg Pathol 26(8):1032–1039
Krishnamurthy S, Dayal Y (1995) Pancreatic metaplasia in Barrett’s esophagus. An immunohistochemical study. Am J Surg Pathol 19(10):1172–1180
el-Zimaity HM et al (2000) The gastric cardia in gastro-oesophageal disease. J Clin Pathol 53(8):619–625
Chlumská A et al (2005) Autoimmune gastritis. A clinicopathologic study of 25 cases. Cesk Patol 41(4):137–42
Jhala NC et al (2003) Pancreatic acinar cell metaplasia in autoimmune gastritis. Arch Pathol Lab Med 127(7):854–857
Johansson J et al (2005) Prevalence of precancerous and other metaplasia in the distal oesophagus and gastro-oesophageal junction. Scand J Gastroenterol 40(8):893–902
Hagiwara T et al (2007) Development of pancreatic acinar cell metaplasia after successful administration of omeprazole for 6 months in rats. Dig Dis Sci 52(5):1219–1224
Matsukawa J et al (2016) Radiographic localization study of a novel potassium-competitive acid blocker, vonoprazan, in the rat gastric mucosa. Dig Dis Sci 61(7):1888–1894
Kubo K et al (2020) Vonoprazan-associated gastric mucosal redness: a report of four cases. Intern Med 59(4):507–511
Mills JC, Goldenring JR (2017) Metaplasia in the stomach arises from gastric chief cells. Cell Mol Gastroenterol Hepatol 4(1):85–88
Leushacke M et al (2017) Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat Cell Biol 19(7):774–786
Burclaff J et al (2020) Proliferation and differentiation of gastric mucous neck and chief cells during homeostasis and injury-induced metaplasia. Gastroenterology 158(3):598-609.e5
Huh WJ et al (2012) Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology 142(1):21-24.e7
Yang YSH et al (2020) Long-term proton pump inhibitor administration caused physiological and microbiota changes in rats. Sci Rep 10(1):866
Sengupta P (2013) The laboratory rat: relating its age with human’s. Int J Prev Med 4(6):624–630
Acknowledgements
Dr. Rodrigo Edelmuth was awarded with the 2022 Career Development Award, provided by the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Rasa Zarnegar works as a consultant for Bard (BD). Dr. Philip Katz works as a consultant for Phathom Pharma. Rodrigo C.L. Edelmuth, Maria Cristina Riascos, Hala Al Asad, Haythem Najah, Jacques A. Greenberg, Ileana C. Miranda, Carl V. Crawford, Felice H Schnoll-Sussman, Philip O. Katz, Brendan M. Finnerty, Thomas J. Fahey III have no conflicts of interest or financial ties to disclose. The Laboratory of Comparative Pathology is supported the NCI Cancer Center Support Grant P30 CA008748.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Edelmuth, R.C.L., Riascos, M.C., Al Asadi, H. et al. Gastric development of pancreatic acinar cell metaplasia after Vonoprazan therapy in rats. Surg Endosc 37, 9366–9372 (2023). https://doi.org/10.1007/s00464-023-10371-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10371-9