Abstract
Background
This study was performed to evaluate the safety and efficacy of laparoscopic gastrectomy (LG) in patients with locally advanced gastric cancer (LAGC) who received neoadjuvant chemotherapy (NACT).
Methods
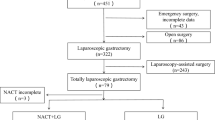
We retrospectively analyzed patients who underwent gastrectomy for LAGC (cT2-4aN+M0) after NACT from January 2015 to December 2019. The patients were divided into a LG group and an open gastrectomy (OG) group. The short- and long-term outcomes in both groups were examined following propensity score matching.
Results
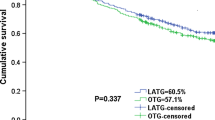
We retrospectively reviewed 288 patients with LAGC who underwent gastrectomy following NACT. Of these 288 patients, 218 were enrolled; after 1:1 propensity score matching, each group comprised 81 patients. The LG group had significantly lower estimated blood loss than the OG group [80 (50–110) vs. 280 (210–320) mL, P < 0.001) but a longer operation time [205 (186.5–222.5) vs. 182 (170–190) min, P < 0.001], a lower postoperative complication rate (24.7% vs. 42.0%, P = 0.002), and a shorter postoperative hospitalization period [8 (7–10) vs. 10 (8–11.5) days, P = 0.001]. Subgroup analysis revealed that patients who underwent laparoscopic distal gastrectomy had a lower rate of postoperative complications than patients in the OG group (18.8% vs. 38.6%, P = 0.034); however, such a pattern was not seen in patients who underwent total gastrectomy (32.3% vs. 45.9%, P = 0.251). The 3-year matched cohort analysis showed no significant difference in overall survival or recurrence-free survival (log-rank P = 0.816 and P = 0.726, respectively) (71.3% and 65.0% in OG vs. 69.1% and 61.7% in LG, respectively).
Conclusion
In the short term, LG following NACT is safer and more effective than OG. However, the long-term results are comparable.
Graphic abstract




Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Wang YLZ, Shan F et al (2018) Current status of diagnosis and treatment of early gastric cancer in China—data from China Gastrointestinal Cancer Surgery Union. Zhonghua Wei Chang Wai Ke Za Zhi 21(2):168–174
Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H (2016) Gastric cancer. The Lancet 388:2654–2664
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJH, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, Participants MT (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11–20
Coccolini F, Nardi M, Montori G, Ceresoli M, Celotti A, Cascinu S, Fugazzola P, Tomasoni M, Glehen O, Catena F, Yonemura Y, Ansaloni L (2018) Neoadjuvant chemotherapy in advanced gastric and esophago-gastric cancer. Meta-analysis of randomized trials. Int J Surg 51:120–127
Al-Batran S-E, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp H-G, Mayer F, Haag GM, Luley K, Lindig U, Schmiegel W, Pohl M, Stoehlmacher J, Folprecht G, Probst S, Prasnikar N, Fischbach W, Mahlberg R, Trojan J, Koenigsmann M, Martens UM, Thuss-Patience P, Egger M, Block A, Heinemann V, Illerhaus G, Moehler M, Schenk M, Kullmann F, Behringer DM, Heike M, Pink D, Teschendorf C, Löhr C, Bernhard H, Schuch G, Rethwisch V, von Weikersthal LF, Hartmann JT, Kneba M, Daum S, Schulmann K, Weniger J, Belle S, Gaiser T, Oduncu FS, Güntner M, Hozaeel W, Reichart A, Jäger E, Kraus T, Mönig S, Bechstein WO, Schuler M, Schmalenberg H, Hofheinz RD (2019) Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. The Lancet 393:1948–1957
Zhang XT, Liang H, Li ZY, Xue YW, Wang YO, Zhou ZW, Yu JR, Bu ZD, Chen L, Du YA, Wang XB, Wu AW, Li GL, Su XQ, Xiao G, Cui M, Wu D, Chen L, Wu XJ, Zhou YB, Zhang LH, Dang CX, He YL, Zhang ZT, Sun YH, Li Y, Chen HQ, Bai YX, Qi CS, Yu PW, Zhu GB, Suo J, Jia BQ, Li LP, Huang CM, Li F, Ye YJ, Xu HM, Wang X, Yuan YN, Jian-Yu E, Ying XJ, Yao C, Shen L, Ji JF, Grp RS (2021) Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol 22:1081–1092
Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, Comm EG (2016) Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 27:v38–v49
NCC Network (2020) NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Gastric Cancer (version 2.2020) 2020
Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, Wang C, Qiu MZ, Cai MY, Wu Q, Liu H, Guan WL, Zhou AP, Zhang YJ, Liu TS, Bi F, Yuan XL, Rao SX, Xin Y, Sheng WQ, Xu HM, Li GX, Ji JF, Zhou ZW, Liang H, Zhang YQ, Jin J, Shen L, Li J, Xu RH (2021) The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun 41:747–795
Kitano S, Iso Y, Moriyama M, Sugimachi K (1994) Laparoscopy-assisted Billroth-I gastrectomy. Surg Laparosc Endosc 4:146–148
Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Hu Y, Liu H, Zheng C, Li P, Xie J, Liu F, Li Z, Zhao G, Yang K, Liu C, Li H, Chen P, Ji J, Li G, Chinese Laparoscopic Gastrointestinal Surgery Study G (2019) Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: the CLASS-01 randomized clinical trial. JAMA 321:1983–1992
Hyung WJ, Yang HK, Park YK, Lee HJ, An JY, Kim W, Kim HI, Kim HH, Ryu SW, Hur H, Kim MC, Kong SH, Cho GS, Kim JJ, Park DJ, Ryu KW, Kim YW, Kim JW, Lee JH, Han SU, Korean Laparoendoscopic G (2020) Long-term outcomes of laparoscopic distal gastrectomy for locally advanced gastric cancer: the KLASS-02-RCT randomized clinical trial. J Clin Oncol 38:3304–3313
Inaki N, Etoh T, Ohyama T, Uchiyama K, Katada N, Koeda K, Yoshida K, Takagane A, Kojima K, Sakuramoto S, Shiraishi N, Kitano S (2015) A multi-institutional, prospective, phase II feasibility study of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for locally advanced gastric cancer (JLSSG0901). World J Surg 39:2734–2741
Xiong BH, Cheng Y, Ma L, Zhang CQ (2014) An updated meta-analysis of randomized controlled trial assessing the effect of neoadjuvant chemotherapy in advanced gastric cancer. Cancer Invest 32:272–284
Li Z, Shan F, Ying X, Zhang Y, Jian-Yu E, Wang Y, Ren H, Su X, Ji J (2019) Assessment of laparoscopic distal gastrectomy after neoadjuvant chemotherapy for locally advanced gastric cancer: a randomized clinical trial. JAMA Surg 154:1093–1101
van der Wielen N, Straatman J, Daams F, Rosati R, Parise P, Weitz J, Reissfelder C, Diez Del Val I, Loureiro C, Parada-Gonzalez P, Pintos-Martinez E, Mateo Vallejo F, Medina Achirica C, Sanchez-Pernaute A, Ruano Campos A, Bonavina L, Asti ELG, Alonso Poza A, Gilsanz C, Nilsson M, Lindblad M, Gisbertz SS, van Berge Henegouwen MI, Fumagalli Romario U, De Pascale S, Akhtar K, Jaap Bonjer H, Cuesta MA, van der Peet DL (2021) Open versus minimally invasive total gastrectomy after neoadjuvant chemotherapy: results of a European randomized trial. Gastr Cancer 24:258–271
Sano T, Coit DG, Kim HH, Roviello F, Kassab P, Wittekind C, Yamamoto Y, Ohashi Y (2017) Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastr Cancer 20:217–225
Japanese Gastric Cancer Association (2021) Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastr Cancer 24:1–21
(2010) NCI common terminology criteria for adverse events (CTCAE) version 4
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid Tumors. J Natl Cancer Inst 92:205–216
Charlson ME, Pompei P, Ales KL, Mackenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
(2021) Protocol for the examination of specimens from patients with carcinoma of the stomach Version: 4.2.1.0
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma, 3rd English edn. Gastric Cancer 14:101–112
Tian YL, Cao SG, Liu XD, Li LP, He QS, Jiang LX, Wang XJ, Chu XQ, Wang H, Xia LJ, Ding YL, Mao WZ, Hui XZ, Shi YR, Zhang HH, Niu ZJ, Li ZQ, Jiang HT, Kehlet H, Zhou YB (2022) Randomized controlled trial comparing the short-term outcomes of enhanced recovery after surgery and conventional care in laparoscopic distal gastrectomy (GISSG1901). Ann Surg 275:E15–E21
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Fujisaki M, Mitsumori N, Shinohara T, Takahashi N, Aoki H, Nyumura Y, Kitazawa S, Yanaga K (2021) Short- and long-term outcomes of laparoscopic versus open gastrectomy for locally advanced gastric cancer following neoadjuvant chemotherapy. Surg Endosc Other Interv Tech 35:1682–1690
Wang NC, Zhou AP, Jin J, Huang H, Zhang YW, Chen YT, Zhao DB (2020) Open vs. laparoscopic surgery for locally advanced gastric cancer after neoadjuvant therapy: Short-term and long-term survival outcomes. Oncol Lett 20:861–867
Yu JH, Wang ZZ, Fan YC, Liu MX, Xu K, Zhang N, Yao ZD, Yang H, Zhang CH, Xing JD, Cui M, Su XQ (2021) Comparison of neoadjuvant chemotherapy followed by surgery vs. surgery alone for locally advanced gastric cancer: a meta-analysis. Chin Med J 134:1669–1680
Robb WB, Messager M, Gronnier C, Tessier W, Hec F, Piessen G, Mariette C, Wo FFET, Chir FFDRE (2015) High-Grade Toxicity to Neoadjuvant Treatment for Upper Gastrointestinal Carcinomas: What is the Impact on Perioperative and Oncologic Outcomes? Ann Surg Oncol 22:3632–3639
D’Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS (2004) Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg 240:808–816
Chou HH, Kuo CJ, Hsu JT, Chen TH, Lin CJ, Tseng JH, Yeh TS, Hwang TL, Jan YY (2013) Clinicopathologic study of node-negative advanced gastric cancer and analysis of factors predicting its recurrence and prognosis. Am J Surg 205:623–630
Nakauchi M, Vos E, Tang LH, Gonen M, Janjigian YY, Ku GY, Ilson DH, Maron SB, Yoon SS, Brennan MF, Coit DG, Strong VE (2021) Outcomes of neoadjuvant chemotherapy for clinical stages 2 and 3 gastric cancer patients: analysis of timing and site of recurrence. Ann Surg Oncol 28:4829–4838
Mokadem I, Dijksterhuis WPM, van Putten M, Heuthorst L, de Vos-Geelen JM, Mohammad NH, Nieuwenhuijzen GAP, van Laarhoven HWM, Verhoeven RHA (2019) Recurrence after preoperative chemotherapy and surgery for gastric adenocarcinoma: a multicenter study. Gastr Cancer 22:1263–1273
Wang F-H, Shen L, Li J, Zhou Z-W, Liang H, Zhang X-T, Tang L, Xin Y, Jin J, Zhang Y-J, Yuan X-L, Liu T-S, Li G-X, Wu Q, Xu H-M, Ji J-F, Li Y-F, Wang X, Yu S, Liu H, Guan W-L, Xu R-H (2019) The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun 39:1–31
van Putten M, Lemmens V, van Laarhoven HWM, Pruijt HFM, Nieuwenhuijzen GAP, Verhoeven RHA (2019) Poor compliance with perioperative chemotherapy for resectable gastric cancer and its impact on survival. Ejso 45:1926–1933
Sedlak K, Rawicz-Pruszynski K, Mlak R, Geca K, Skorzewska M, Pelc Z, Malecka-Massalska T, Polkowski WP (2022) Union is strength: textbook outcome with perioperative chemotherapy compliance decreases the risk of death in advanced gastric cancer patients. EJSO 48:356–361
Acknowledgements
The authors are grateful to Prof. Xiaobin Zhou from the Department of Epidemiology and Health Statistics, Qingdao University, for his great technical assistance throughout the experiment’s design and data processing. The authors also thank Angela Morben, DVM, ELS, from Liwen Bianji (Edanz) (www.liwenbianji.cn), for editing the English text of a draft of this manuscript.
Funding
This work was supported by the Shandong Provincial Natural Science Foundation, China (No. ZR2021MH001).
Author information
Authors and Affiliations
Contributions
HZ: data acquisition, analysis, and interpretation; manuscript drafting. XL: data acquisition and interpretation. YT: data acquisition and interpretation. SC: data acquisition. ZL: data acquisition. GL: manuscript revision, data interpretation. YS: data acquisition. XZ: data acquisition. ZH: data acquisition. CM: data acquisition. ZJ: data acquisition. QW: data acquisition. YZ: study conception and design, data interpretation, manuscript revision.
Corresponding author
Ethics declarations
Disclosure
Hao Zhong, Xiaodong Liu, Yulong Tian, Shougen Cao, Zequn Li, Gan Liu, Yuqi Sun, Xingqi Zhang, Zhenlong Han, Cheng Meng, Zhuoyu Jia, Qingrui Wang, and Yanbing Zhou have no conflicts of interest or financial ties to disclose.
Ethical approval
The study was approved by the Affiliated Hospital of Qingdao University ethics review committee (Approval Number QYFYWZLL27017). All procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhong, H., Liu, X., Tian, Y. et al. Comparison of short- and long-term outcomes between laparoscopic and open gastrectomy for locally advanced gastric cancer following neoadjuvant chemotherapy: a propensity score matching analysis. Surg Endosc 37, 5902–5915 (2023). https://doi.org/10.1007/s00464-023-10052-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10052-7