Abstract
Introduction
The mission of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) is to innovate, educate, and collaborate to improve patient care. A critical element in meeting this mission is the publishing of trustworthy and current guidelines for the practicing surgeon.
Methods
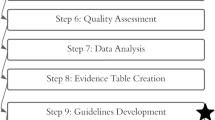
In this manuscript, we outline the steps of developing high quality practice guidelines using a completely volunteer-based professional organization.
Results
SAGES has developed a standardized approach to train volunteer surgeons and trainees alike to develop clinically pertinent guidelines in a timely manner, without sacrificing quality.
Conclusions
This methodology can be used more widely by volunteer organizations to efficiently develop effective tools for practicing physicians.


Similar content being viewed by others
References
Moher D, Tetzlaff J, Tricco AC, Sampson M, Altman DG (2007) Epidemiology and reporting characteristics of systematic reviews. PLoS Med 4:e78
World Health Organization (WHO) (2010) WHO handbook for guideline development. WHO, International Repository for Information Sharing, Geneva
Institute of Medicine (2011) Clinical practice guidelines we can trust: standards for developing trustworthy clinical practice guidelines (CPGs). The National Academies Press, Washington, DC
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ, Group GW (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336:924–926
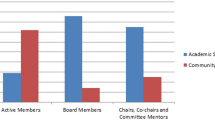
Dirks RC, Walsh D, Haggerty S, Kohn GP, Pryor A, Stefanidis D (2021) SAGES guidelines: an appraisal of their quality and value by SAGES members. Surg Endosc. https://doi.org/10.1007/s00464-021-08323-2
Wallace BC, Small K, Brodley CE, Lau J, Trikalinos TA (2012) Deploying an interactive machine learning system in an evidence-based practice center: abstrackr. In: Proceedings of the 2nd ACM SIGHIT International Health Informatics Symposium, Association for Computing Machinery, Miami, Florida, pp 819–824
Covidence systematic review software (2021). Veritas Health Innovation, Melbourne, Australia. http://www.covidence.org. Accessed 16 April 2021
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernan MA, Hopewell S, Hrobjartsson A, Junqueira DR, Juni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P (2019) The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Ottawa Hospital, Ottawa
Review Manager (RevMan) (2014) Software. The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen
GRADEpro GDT: GRADEpro Guideline Development Tool [Software] (2020). McMaster University, 2015 (developed by Evidence Prime, Inc.). https://gradepro.org. Accessed 12 April 2021
Brunt ML, Deziel DJ, Telem DA, Strasberg SM, Aggarwal R, Asbun H, Bonjer J, McDonald M, Alseidi A, Ujiki M, Riall TS, Hammill C, Moulton CA, Pucher PH, Parks RW, Ansari MT, Connor S, Dirks RC, Anderson B, Altieri MS, Tsamalaidze L, Stefanidis D, Prevention of Bile Duct Injury Consensus Work Group (2020) Safe cholecystectomy multi-society practice guideline and state-of-the-art consensus conference on prevention of bile duct injury during cholecystectomy. Surg Endosc 34:2827–2855
Kohn GP, Dirks RC, Ansari MT, Clay J, Dunst CM, Lundell L, Marks JM, Molena D, Rooker C, Saxena P, Swanstrom L, Wong RK, Pryor AD, Stefanidis D (2021) Guidelines for the use of peroral endoscopic myotomy (POEM) for the treatment of achalasia. Surg Endosc online ahead of print
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA (2017) AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 358:j4008
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Amelia T. Rogers, MD—salary support from SAGES for role as Guidelines Fellow. Rebecca Dirks, MD, MS—SAGES paid for travel to the G-I-N (guidelines international network) conference last year; hold stock in Johnson and Johnson, unrelated to current project. Holly Ann Burt, MLIS—contracted by SAGES as a medical librarian. Geoffrey P. Kohn, MBBS, MSurg—paid by Avant Law Australia to provide expert opinion reports in the field of upper GI surgery, unrelated to this manuscript. Bethany J. Slater, MD, MBA—receives consulting fees from Boulder Surgical. Danielle Walsh, MD—unpaid member of Society of Gastrointestinal and Endoscopic Surgeons Board of Governors—member at large; American College of Surgeons Board of Governors; American Academy of Pediatrics Surgery Committee. Aurora Pryor, MD, MBA—receives honoraria for speaking from Ethicon, Gore, Medtronic, Merck, and Stryker. Stephen Haggerty, MD and Dimitrios Stefanidis, MD, PhD—have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
Databases used for literature search
Primary resources
-
1.
MEDLINE
-
MEDLINE consists of biomedical and life sciences citations indexed by the National Library of Medicine (NLM) and includes 27 million citations from 5274 regularly indexed journals using MeSH (Medical Subject Headings)
-
PubMed (http://pubmed.gov/): Free search interface created by the NLM searches 31 million indexed and non-indexed citations from over 37,500 journals (1781-present)
-
Ovid MEDLINE (https://www.ovid.com/) (1946-present) and EBSCO MEDLINE (https://www.ebsco.com/products/research-databases/medline) (1809-present) are subscription interfaces using NLM data
-
-
2.
Embase (https://www.embase.com/)
-
Subscription database of 32 million records of biomedical and pharmacological articles (1947-present) and conference abstracts (2009-present)
-
Search 8500 journals including MEDLINE titles and 2900 uniquely indexed using the EMTREE indexing system
-
-
3.
Cochrane library (https://www.cochranelibrary.com/)
-
Focused on informing healthcare decision making, the free search interface accesses nearly 1.7 million items from six databases including Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR)
-
Search results include trials (from Clinicaltrials.gov and ICTRP), articles (Embase, PubMed, CINAHL), and Cochrane publications (reviews, protocols, etc.); some items are available only by subscription
-
-
4.
Clinicaltrials.gov (https://clinicaltrials.gov)
-
Nearly 350,000 freely accessible clinical trials in all phases from 216 countries: search by disease/condition, intervention, or other terms (2000-present)
-
Trials may include related article citations linked to PubMed (either supporting articles or publications of results) and/or raw study results
-
-
5.
ICTRP: International Clinical Trials Registry Platform (https://www.who.int/ictrp/en/)
-
Over 500,000 freely accessible registered trials with links to individual country clinical trial platforms but few linked articles or study results (2005-present)
-
Use the trial ID(s) to search Google Scholar and/or PubMed for related articles
-
Specialty databases
-
1.
CINAHL: Cumulative Index to Nursing and Allied Health Literature (https://www.ebscohost.com/nursing/products/cinahl-databases)
-
Subscription databases focused on nursing, alternative/complementary medicine, biomedicine, and physical and occupational therapy. Search 760–5,500 journals (depending on product) indexed with CINAHL Subject Headings (1937-present)
-
-
2.
PsycINFO (https://www.apa.org/pubs/databases/psycinfo/)
-
American Psychological Association (APA) subscription database focused on social, psychological, and behavioral sciences. Search 4.9 million articles and book chapters indexed with the APA Thesaurus of Psychological Index Terms (1700s-present)
-
-
3.
LILACS: Latin American and Caribbean Health Sciences Literature (https://lilacs.bvsalud.org/en/)
-
WHO PAHO (World Health Organization Pan-American Health Organization) offers this free search interface of nearly 1 million health science records from 900 journals published in Latin America and Caribbean countries and indexed with Descriptors in Health Sciences (DeCS), an extended translation of MeSH. Languages: Portuguese, Spanish, and English (1982-present)
-
-
4.
ERIC: Education Resources Information Center (https://eric.ed.gov/)
-
This free Institute of Education Services sponsored database includes 1.7 million records focused on education research from 1200 journals and 700 government and non-profit sources. Descriptor and identification terms added. (1966-present). ERIC (EBSCO) and ERIC (ProQuest) are subscription databases accessing ERIC data
-
-
5.
Theses and dissertations
-
Open Access Theses and Dissertations (OATD) (https://oatd.org/): Free search of over 5.1 million publications from 1100 international institutions (1734-present)
-
ProQuest Dissertations and Theses Global (https://proquest.libguides.com/pqdt) Subscription database searching over 5 million international records (1637-present)
-
-
6.
International HTA (Health Technology Assessment) Database (https://database.inahta.org/)
-
Free interface searching 17,000 HTAs indexed with MeSH and providing links to the published reports (1989-Present)
-
-
7.
FDA Manufacturer and User Facility Device Experience (MAUDE) database (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/search.CFM)
-
This Food and Drug Administration (FDA) database contains over 9.2 million reports on medical devices that malfunctioned or caused death or serious injury submitted by manufacturers, voluntary reporters. Related databases are linked (1993-present)
-
Additional resources and portals
-
1.
Turning research into practice (TRIP) medical database (https://www.tripdatabase.com/)
-
Free search engine focused on clinical research retrieving over 2.5 million articles from free online resources and sorted by study type. Optional subscription includes additional clinical trials, medical images, and systematic reviews
-
-
2.
Virtual health library (VHL) regional portal: https://bvsalud.org/en/
-
Sponsored by WHO PAHO, this free interface searches across multiple Pan-American databases including LILACS, Bibliographic Index Spanish in Health Sciences (IBECS) and the National Bibliography of Health Sciences (BINACIS)
-
-
3.
DOAJ: directory of open access journals (https://doaj.org/)
-
A free portal searching 15,000 international and interdisciplinary peer reviewed journals and over 5.1 million articles from 133 countries. Subject indexing added to journals which must meet strict guidelines to remain included (1874-present)
-
-
4.
Google scholar (https://scholar.google.com/)
-
Free interface searching freely accessible scholarly literature across the web including online journal citations, citation services (e.g., PubMed), pre-print servers, and patents. No indexing; uses only author explicit terms. Automated citation analysis
-
-
5.
Web of science (https://clarivate.com/webofsciencegroup/)
-
Subscription database searching 171 million records from 34,600 journals in the natural and biomedical sciences. Provides links to items citing a selected article (times cited), the article’s cited references, and author h-indexes (1800-present)
-
-
6.
Scopus (https://www-elsevier-com.proxy.ulib.uits.iu.edu/solutions/scopus)
-
Subscription database focused on sciences and social sciences. Search over 75 million records from 38,000 journals, book chapters, and conference papers. Provides links to items citing a selected article (cited by) and the article’s references (1788-present)
-
-
7.
AHRQ: agency for healthcare research and quality (https://www.ahrq.gov/)
-
AHRQ collects data and publications in multiple topic areas; databases include Systematic Review Data Repository (SRDR), Medical Expenditure Panel Survey (MEPS), and National Healthcare Quality and Disparities Reports
-
Medical libraries may provide access to subscription databases.
Appendix 2
Modified Newcastle–Ottawa Scale


Rights and permissions
About this article
Cite this article
Rogers, A.T., Dirks, R., Burt, H.A. et al. Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) guidelines development: standard operating procedure. Surg Endosc 35, 2417–2427 (2021). https://doi.org/10.1007/s00464-021-08469-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-021-08469-z