Abstract
Introduction
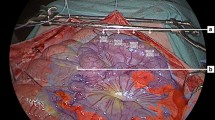
The use of Indocyanine green (ICG) fluorescence angiography (ICG-FA) is an applied method to assess visceral perfusion during surgical procedures worldwide. Further development has entailed quantification of the fluorescence signal; however, whether quantified ICG-FA can detect intraoperative changes in perfusion after hemorrhage has not been investigated previously. In this study, we investigated whether a quantification method, developed and validated in our department (q-ICG), could detect changes in gastric perfusion induced by hemorrhage and resuscitation.
Methods
Ten pigs were included in the study. Specific regions of interest of the stomach were chosen, and three q-ICG measurements of gastric perfusion obtained: 20 min after completion of the laparoscopic setup (baseline), after reducing the circulating blood volume by 30%, and after reinfusion of the withdrawn blood volume. Hemodynamic variables were recorded, and blood samples were collected every 10 min during the procedure.
Results
The reduction in blood volume generated decreased gastric perfusion (q-ICG) from baseline (p = 0.023), and gastric perfusion subsequently increased (p < 0.001) after the reintroduction of the withdrawn blood volume. Cardiac output (CO) and mean arterial blood pressure (MAP) shifted correspondingly and the gastric perfusion correlated to CO (r = 0.575, p = 0.001) and MAP (r = 0.436, p = 0.018).
Conclusion
We present a novel study showing that the q-ICG method can detect dynamic changes in local tissue perfusion induced by hemorrhage and resuscitation. As regional gastrointestinal perfusion may be significantly reduced, while hemodynamic variables such as MAP or heart rate remain stable, q-ICG may provide an objective, non-invasive method for detecting regional early ischemia, strengthening surgical decision making.




Similar content being viewed by others
References
Bischoff PM, Niederberger HJ, Török B, Speiser P (1995) Simultaneous indocyanine green and fluorescein angiography. Retina 15(2):91–99
Alander JT, Kaartinen I, Laakso A, Pätilä T, Spillmann T, Tuchin VV, Venermo M, Välisuo P (2012) A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 2012:1–26
Mangano A, Fernandes E, Gheza F, Bustos R, Chen LL, Masrur M, Giulianotti PC (2019) Near-infrared indocyanine green-enhanced fluorescence and evaluation of the bowel microperfusion during robotic colorectal surgery: a retrospective original paper. Surg Technol Int 34:93–100
Zehetner J, DeMeester SR, Alicuben ET, Oh DS, Lipham JC, Hagen JA, DeMeester TR (2015) Intraoperative assessment of perfusion of the gastric graft and correlation with anastomotic leaks after esophagectomy. Ann Surg 262(1):74–78
Mangano A, Gheza F, Chen LL, Minerva EM, Giulianotti PC (2018) Indocyanine green (Icg)-enhanced fluorescence for intraoperative assessment of bowel microperfusion during laparoscopic and robotic colorectal surgery: the quest for evidence-based results. Surg Technol Int 32:101–104
Gossedge G, Vallance A, Jayne D (2015) Diverse applications for near infra-red intraoperative imaging. Color Dis 17:7–11
Granger DN, Kvietys PR (2003) The splanchnic circulation: intrinsic regulation. Annu Rev Physiol 43(1):409–418
Krejci V, Hiltebrand L, Banic A, Erni D, Wheatley AM, Sigurdsson GH (2000) Continuous measurements of microcirculatory blood flow in gastrointestinal organs during acute haemorrhage. Br J Anaesth 84(4):468–475
Harper D, Chandler B (2016) Splanchnic circulation. BJA Educ 16(2):66–71
Edouard AR, Degrémont AC, Duranteau J, Pussard E, Berdeaux A, Samii K (1994) Heterogeneous regional vascular responses to simulated transient hypovolemia in man. Intensive Care Med 20(6):414–420
Riddez L, Hahn RG, Brismar B, Strandberg A, Svensén C, Hedenstierna G (1997) Central and regional hemodynamics during acute hypovolemia and volume substitution in volunteers. Crit Care Med 25(4):635–640
Jakob SM (2003) Splanchnic blood flow in low-flow states. Anesth Analg 96(4):1129–1138
Pommergaard H-C, Achiam MP, Burcharth J, Rosenberg J (2015) Impaired blood supply in the colonic anastomosis in mice compromises healing. Int Surg 100(1):70–76
Kruschewski M, Rieger H, Pohlen U, Hotz HG, Buhr HJ (2007) Risk factors for clinical anastomotic leakage and postoperative mortality in elective surgery for rectal cancer. Int J Colorectal Dis 22(8):919–927
Kim MJ, Shin R, Oh H-K, Park JW, Jeong S-Y, Park J-G (2011) The impact of heavy smoking on anastomotic leakage and stricture after low anterior resection in rectal cancer patients. World J Surg 35(12):2806–2810
Fawcett A, Shembekar M, Church JS, Vashisht R, Springall RG, Nott DM (1996) Smoking, hypertension, and colonic anastomotic healing; a combined clinical and histopathological study. Gut 38(5):714–718
Kassis ES, Kosinski AS, Ross P, Koppes KE, Donahue JM, Daniel VC (2013) Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg 96(6):1919–1926
Watanabe J, Ishibe A, Suwa Y, Suwa H, Ota M, Kunisaki C, Endo I (2020) Indocyanine green fluorescence imaging to reduce the risk of anastomotic leakage in laparoscopic low anterior resection for rectal cancer: a propensity score-matched cohort study. Surg Endosc 34(1):202–208
Sujatha-Bhaskar S, Jafari MD, Stamos MJ (2017) The role of fluorescent angiography in anastomotic leaks. Surg Technol Int 30:83–88
Alekseev M, Rybakov E, Shelygin Y, Chernyshov S, Zarodnyuk I (2020) A study investigating the perfusion of colorectal anastomoses using fluorescence angiography: results of the FLAG randomized trial. Color Dis. https://doi.org/10.1111/codi.15037
Ladak F, Dang JT, Switzer N, Mocanu V, Tian C, Birch D, Turner SR, Karmali S (2019) Indocyanine green for the prevention of anastomotic leaks following esophagectomy: a meta-analysis. Surg Endosc 33(2):384–394
Nerup N, Svendsen MBS, Svendsen LB, Achiam MP (2020) Feasibility and usability of real-time intraoperative quantitative fluorescent-guided perfusion assessment during resection of gastroesophageal junction cancer. Langenbeck’s Arch Surg 405(2):215–222
Wada T, Kawada K, Takahashi R, Yoshitomi M, Hida K, Hasegawa S, Sakai Y (2017) ICG fluorescence imaging for quantitative evaluation of colonic perfusion in laparoscopic colorectal surgery. Surg Endosc 31(10):4184–4193
Ishige F, Nabeya Y, Hoshino I, Takayama W, Chiba S, Arimitsu H, Iwatate Y, Yanagibashi H (2019) Quantitative assessment of the blood perfusion of the gastric conduit by indocyanine green imaging. J Surg Res 234:303–310
Son GM, Kwon MS, Kim Y, Kim J, Kim SH, Lee JW (2019) Quantitative analysis of colon perfusion pattern using indocyanine green (ICG) angiography in laparoscopic colorectal surgery. Surg Endosc 33(5):1640–1649
Nerup N, Knudsen KBK, Ambrus R, Svendsen MBS, Thymann T, Ifaoui IBR, Svendsen LB, Achiam MP (2017) Reproducibility and reliability of repeated quantitative fluorescence angiography. Surg Technol Int 31:35–39
Nerup N, Andersen HS, Ambrus R, Strandby RB, Svendsen MBS, Madsen MH, Svendsen LB, Achiam MP (2017) Quantification of fluorescence angiography in a porcine model. Langenbeck’s Arch Surg 402(4):655–662
Nerup N, Ring LL, Strandby RB, Egeland C, Svendsen MBS, Hasselby JP, Willemoe GL, Hartmann B, Svendsen LB, Achiam MP (2018) Quantitative perfusion assessment of intestinal anastomoses in pigs treated with glucagon-like peptide 2. Langenbeck’s Arch Surg 403(7):881–889
Rønn JH, Nerup N, Strandby RB, Svendsen MBS, Ambrus R, Svendsen LB, Achiam MP (2019) Laser speckle contrast imaging and quantitative fluorescence angiography for perfusion assessment. Langenbeck’s Arch Surg 404(4):505–515
Diana M, Noll E, Diemunsch P, Dallemagne B, Benahmed MA, Agnus V, Soler L, Barry B, Namer IJ, Demartines N, Charles AL, Geny B, Marescaux J (2014) Enhanced-reality video fluorescence: a real-time assessment of intestinal viability. Ann Surg 259(4):700–707
Diana M, Halvax P, Dallemagne B, Nagao Y, Diemunsch P, Charles AL, Agnus V, Soler L, Demartines N, Lindner V, Geny B, Marescaux J (2014) Real-time navigation by fluorescence-based enhanced reality for precise estimation of future anastomotic site in digestive surgery. Surg Endosc 28(11):3108–3118
Diana M, Agnus V, Halvax P, Liu YY, Dallemagne B, Schlagowski AI, Geny B, Diemunsch P, Lindner V, Marescaux J (2015) Intraoperative fluorescence-based enhanced reality laparoscopic real-time imaging to assess bowel perfusion at the anastomotic site in an experimental model. Br J Surg 102(2):169–176
Diana M, Dallemagne B, Chung H, Nagao Y, Halvax P, Agnus V, Soler L, Lindner V, Demartines N, Diemunsch P, Geny B, Swanström L, Marescaux J (2014) Probe-based confocal laser endomicroscopy and fluorescence-based enhanced reality for real-time assessment of intestinal microcirculation in a porcine model of sigmoid ischemia. Surg Endosc 28(11):3224–3233
D’Urso A, Agnus V, Barberio M, Seeliger B, Marchegiani F, Charles AL, Geny B, Marescaux J, Mutter D, Diana M (2020) Computer-assisted quantification and visualization of bowel perfusion using fluorescence-based enhanced reality in left-sided colonic resections. Surg Endosc. https://doi.org/10.1007/s00464-020-07922-9
Amagai H, Miyauchi H, Muto Y, Uesato M, Ohira G, Imanishi S, Maruyama T, Tochigi T, Okada K, Maruyama M, Matsubara H (2019) Clinical utility of transanal indocyanine green near-infrared fluorescence imaging for evaluation of colorectal anastomotic perfusion. Surg Endosc 34(12):5283–5293
Hayami S, Matsuda K, Iwamoto H, Ueno M, Kawai M, Hirono S, Okada K, Miyazawa M, Tamura K, Mitani Y, Kitahata Y, Mizumoto Y, Yamaue H (2019) Visualization and quantification of anastomotic perfusion in colorectal surgery using near-infrared fluorescence. Tech Coloproctol 23(10):973–980
Kamiya K, Unno N, Miyazaki S, Sano M, Kikuchi H, Hiramatsu Y, Ohta M, Yamatodani T, Mineta H, Konno H (2015) Quantitative assessment of the free jejunal graft perfusion. J Surg Res 194(2):394–399
Iwamoto H, Matsuda K, Hayami S, Tamura K, Mitani Y, Mizumoto Y, Nakamura Y, Murakami D, Ueno M, Yokoyama S, Hotta T, Takifuji K, Yamaue H (2020) Quantitative indocyanine green fluorescence imaging used to predict anastomotic leakage focused on rectal stump during laparoscopic anterior resection. J Laparoendosc Adv Surg Tech 30(5):542–546
Cohn SM, Varela JE, Giannotti G, Dolich MO, Brown M, Feinstein A, McKenney MG, Spalding P (2001) Splanchnic perfusion evaluation during hemorrhage and resuscitation with gastric near-infrared spectroscopy. J Trauma 50(4):629–635
Kivilaakso E, Ahonen J, Aronsen KF, Höckerstedt K, Kalima T, Lempinen M, Suoranta H, Vernerson E (1982) Gastric blood flow, tissue gas tension and microvascular changes during hemorrhage-induced stress ulceration in the pig. Am J Surg 143(3):322–330
Varela JE, Cohn SM, Diaz I, Giannotti GD, Proctor KG (2003) Splanchnic perfusion during delayed, hypotensive, or aggressive fluid resuscitation from uncontrolled hemorrhage. Shock 20(5):476–480
Jakob SM, Tenhunen JJ, Laitinen S, Heino A, Alhava E, Takala J (2001) Effects of systemic arterial hypoperfusion on splanchnic hemodynamics and hepatic arterial buffer response in pigs. Am J Physiol Gastrointest Liver Physiol 280(5):G819–G827
Krogh A (1929) The progress of physiology. Am J Physiol Content 90(2):243–251
Miwa M (2010) The principle of ICG fluorescence method [Internet]. Open Surg Oncol J 2:26–28
Secher NH, Jacobsen J, Friedman DB, Matzen S (1992) Bradycardia during reversible hypovolaemic shock: associated neural reflex mechanisms and clinical implications. Clin Exp Pharmacol Physiol 19(11):733–743
Østergaard G, Nordahl-Hansen H, Lund-Ottesen J (2011) Physiological, hematological, and clinical chemistry parameters, including conversion factors. In: Hau J, Schapiro S (eds) Handbook of laboratory animal science. CRC Press, Boca Raton, FL, pp 667–707
Draijer M, Hondebrink E, van Leeuwen T, Steenbergen W (2009) Review of laser speckle contrast techniques for visualizing tissue perfusion. Lasers Med Sci 24(4):639–651
Reinhardt CP, Dalhberg S, Tries MA, Marcel R, Leppo JA (2001) Stable labeled microspheres to measure perfusion: validation of a neutron activation assay technique. Am J Physiol Heart Circ Physiol 280(1):H108–H116
Davis MA, Kazmi SMS, Dunn AK (2014) Imaging depth and multiple scattering in laser speckle contrast imaging. J Biomed Opt 19(8):86001
Alemanno G, Somigli R, Prosperi P, Bergamini C, Maltinti G, Giordano A, Valeri A (2016) Combination of diagnostic laparoscopy and intraoperative indocyanine green fluorescence angiography for the early detection of intestinal ischemia not detectable at CT scan. Int J Surg Case Rep 26:77–80
Lütken CD, Achiam MP, Osterkamp J, Svendsen MB, Nerup N (2020) Quantification of fluorescence angiography: toward a reliable intraoperative assessment of tissue perfusion—a narrative review. Langenbeck’s Arch Surg. https://doi.org/10.1007/s00423-020-01966-0
Lütken CD, Achiam MP, Svendsen MB, Boni L, Nerup N (2020) Optimizing quantitative fluorescence angiography for visceral perfusion assessment [Internet]. Surg Endosc 34:5223–5233
Price HL, Deutsch S, Marshall BE, Stephen GW, Behar MG, Neufeld GR (1966) Hemodynamic and metabolic effects of hemorrhage in man, with particular reference to the splanchnic circulation. Circ Res 18(5):469–474
McNeill JR (1971) Role of vasopressin and angiotensin in response of splanchnic resistance vessels to hemorrhage. Adv Exp Med Biol 23:127–144
Wilson C, Gupta R, Gilmour DG, Imrie CW (1987) Acute superior mesenteric ischaemia. Br J Surg 74(4):279–281
Hamilton-Davies C, Mythen MG, Salmon JB, Jacobson D, Shukla A, Webb AR (1997) Comparison of commonly used clinical indicators of hypovolaemia with gastrointestinal tonometry. Intensive Care Med 23(3):276–281
Woolsey CA, Coopersmith CM (2006) Vasoactive drugs and the gut: is there anything new? Curr Opin Crit Care 12(2):155–159
Brinkmann A, Seeling W, Wolf CF, Kneitinger E, Schönberger C, Vogt N, Orend KH, Büchler M, Radermacher P, Georgieff M (1998) Vasopressor hormone response following mesenteric traction during major abdominal surgery. Acta Anaesthesiol Scand 42(8):948–956
Brinkmann A, Seeling W, Rockemann M, Junge JH, Radermacher P, Wiedeck H, Büchler MW, Georgieff M (1999) Changes in gastric intramucosal pH following mesenteric traction in patients undergoing pancreas surgery. Dig Surg 16(2):117–124
Fujimoto Y, Nomura Y, Hirakawa K, Hotta A, Nakamoto A, Yoshikawa N, Ohira N, Tatekawa S (2012) Flurbiprofen axetil provides a prophylactic benefit against mesenteric traction syndrome associated with remifentanil infusion during laparotomy. J Anesth 26(4):490–495
Couto AH, Siqueira H, Brasileiro PP, Cavalcanti IL, Videira da RLR (2017) Severe intraoperative shock related to mesenteric traction syndrome. A A Case Rep 8(3):51–54
Kirton OC, Windsor J, Wedderburn R, Hudson-Civetta J, Shatz DV, Mataragas NR, Civetta JM (1998) Failure of splanchnic resuscitation in the acutely injured trauma patient correlates with multiple organ system failure and length of stay in the ICU. Chest 113(4):1064–1069
Doglio GR, Pusajo JF, Egurrola MA, Bonfigli GC, Parra C, Vetere L, Hernandez MS, Fernandez S, Palizas F, Gutierrez G (1991) Gastric mucosal pH as a prognostic index of mortality in critically ill patients. Crit Care Med 19(8):1037–1040
Ambrus R, Svendsen LB, Secher NH, Goetze JP, Rünitz K, Achiam MP (2017) Severe postoperative complications may be related to mesenteric traction syndrome during open esophagectomy. Scand J Surg 106(3):241–248
Olsen AA, Strandby RB, Nerup N, Ambrus R, Gøtze JP, Svendsen LB, Achiam MP (2020) Development of a severe mesenteric traction syndrome during major abdominal surgery is associated with increased postoperative morbidity: secondary data analysis on prospective cohorts. Langenbeck’s Arch Surg 405(1):81–90
Karliczek A, Harlaar NJ, Zeebregts CJ, Wiggers T, Baas PC, van Dam GM (2009) Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int J Colorectal Dis 24(5):569–576
Karampinis I, Keese M, Jakob J, Stasiunaitis V, Gerken A, Attenberger U, Post S, Kienle P, Nowak K (2018) Indocyanine green tissue angiography can reduce extended bowel resections in acute mesenteric ischemia. J Gastrointest Surg 22(12):2117–2124
Acknowledgements
We thank Anders Bech Jørgensen, Andreas Arendtsen Rostved, Karina Adler Riemenschneider Mikkel Marquard Jessen, August Olsen, Olivia Mortensen and Søren Roepstorff (Department of Surgical Gastroenterology, Rigshospitalet, University Hospital of Copenhagen) for their excellent operative assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Jens Osterkamp, Rune Strandby, Nikolaj Nerup, Morten Bo Søndergaard Svendsen, Lars Svendsen, Michael P. Achiam have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Osterkamp, J., Strandby, R., Nerup, N. et al. Quantitative fluorescence angiography detects dynamic changes in gastric perfusion. Surg Endosc 35, 6786–6795 (2021). https://doi.org/10.1007/s00464-020-08183-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-08183-2