Abstract
Background
Laparoscopic lateral pelvic lymph node dissection (LPLND) in rectal cancer surgery requires considerable skill because the pelvic arteries, which need to be located to guide the dissection, are covered by other tissues and cannot be observed on laparoscopic views. Therefore, surgeons need to localize the pelvic arteries accurately before dissection, to prevent injury to these arteries.
Methods
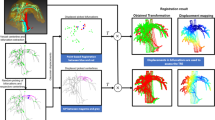
This report proposes a surgical navigation system to facilitate artery localization in laparoscopic LPLND by combining ultrasonic imaging and laparoscopy. Specifically, free-hand laparoscopic ultrasound (LUS) is employed to capture the arteries intraoperatively in this approach, and a laparoscopic vision-based tracking system is utilized to track the LUS probe. To extract the artery contours from the two-dimensional ultrasound image sequences efficiently, an artery extraction framework based on local phase-based snakes was developed. After reconstructing the three-dimensional intraoperative artery model from ultrasound images, a high-resolution artery model segmented from preoperative computed tomography (CT) images was rigidly registered to the intraoperative artery model and overlaid onto the laparoscopic view to guide laparoscopic LPLND.
Results
Experiments were conducted to evaluate the performance of the vision-based tracking system, and the average reconstruction error of the proposed tracking system was found to be 2.4 mm. Then, the proposed navigation system was quantitatively evaluated on an artery phantom. The reconstruction time and average navigation error were 8 min and 2.3 mm, respectively. A navigation system was also successfully constructed to localize the pelvic arteries in laparoscopic and open surgeries of a swine. This demonstrated the feasibility of the proposed system in vivo. The construction times in the laparoscopic and open surgeries were 14 and 12 min, respectively.
Conclusions
The experimental results showed that the proposed navigation system can guide laparoscopic LPLND and requires a significantly shorter setting time than the state-of-the-art navigation systems do.










Similar content being viewed by others
References
Cancer Registry and Statistics (2012) Cancer information service. National Cancer Center, Japan
Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Ishihara S (2015) Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 20(2):207–239
Nakamura T, Watanabe M (2013) Lateral lymph node dissection for lower rectal cancer. World J Surg 37(8):1808–1813
Sadahiro S, Suzuki T, Ishikawa K, Nakamura T, Tanaka Y, Masuda T, Murayama C (2003) Recurrence patterns after curative resection of colorectal cancer in patients followed for a minimum of ten years. Hepatogastroenterology 50(53):1362–1366
Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, Chang HJ, Lee HS, Kim SY, Jung KH, Hong YS, Kim JH, Sohn DK, Kim DH, Oh JH (2010) Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 11(7):637–645
Tonutti M, Elson DS, Yang GZ, Darzi AW, Sodergren MH (2016) The role of technology in minimally invasive surgery: state of the art, recent developments, and future directions. Postgrad Med J 93(1097):159–167
Atallah S, Martin-Perez B, Larach S (2015) Image-guided real-time navigation for transanal total mesorectal excision: a pilot study. Tech Coloproctol 19(11):679–684
Atallah S, Larach SW, Monson JR (2016) Stereotactic navigation for TAMIS-TME. Minim Invasive Ther Allied Technol 25(5):271–277
Koivukangas T, Katisko JP, Koivukangas JP (2013) Technical accuracy of optical and electromagnetic tracking systems. SpringerPlus 2(1):90
Nijkampa J, Kuhlmanna K, Sonkeb JJ, Ruersa T (2016) Image-guided navigation surgery for pelvic malignancies using electromagnetic tracking. SPIE Med Imaging. https://doi.org/10.1117/12.2216213
Li FP, Rajchl M, White JA, Goela A, Peters TM (2015) Ultrasound guidance for beating heart mitral valve repair augmented by synthetic dynamic CT. IEEE Trans Med Imaging 34(10):2025–2035
Rivaz H, Chen SJS, Collins DL (2015) Automatic deformable MR-ultrasound registration for image-guided neurosurgery. IEEE Trans Med Imaging 34(2):366–380
Simpfendörfer T, Baumhauer M, Müller M, Gutt CN, Meinzer HP, Rassweiler JJ, Guven S, Teber D (2011) Augmented reality visualization during laparoscopic radical prostatectomy. J Endourol 25(12):1841–1845
Sridhar AN, Hughes-Hallett A, Mayer EK, Pratt PJ, Edwards PJ, Yang GZ, Darzi AW, Vale JA (2013) Image-guided robotic interventions for prostate cancer. Nat Rev Urol 10(8):452–462
Baumhauer M, Feuerstein M, Meinzer HP, Rassweiler J (2008) Navigation in endoscopic soft tissue surgery: perspectives and limitations. J Endourol 22(4):751–766
Langø T, Vijayan S, Rethy A, Våpenstad C, Solberg OV, Mårvik R, Johnsen G, Hernes TN (2012) Navigated laparoscopic ultrasound in abdominal soft tissue surgery: technological overview and perspectives. Int J Comput Assist Radiol Surg 7(4):585–599
Penney GP, Blackall JM, Hamady MS, Sabharwal T, Adam A, Hawkes DJ (2004) Registration of freehand 3D ultrasound and magnetic resonance liver images. Med Image Anal 8(1):81–91
Konishi K, Nakamoto M, Kakeji Y, Tanoue K, Kawanaka H, Yamaguchi S, Ieiri S, Sato Y, Maehara Y, Tamura S, Hashizume M (2007) A real-time navigation system for laparoscopic surgery based on three-dimensional ultrasound using magneto-optic hybrid tracking configuration. Int J Comput Assist Radiol Surg 2(1):1–10
Song Y, Totz J, Thompson S, Johnsen S, Barratt D, Schneider C, Gurusamy K, Davidson B, Ourselin S, Hawkes D, Clarkson MJ (2015) Locally rigid, vessel-based registration for laparoscopic liver surgery. Int J Comput Assist Radiol Surg 10(12):1951–1961
Sánchez-Margallo JA, Langø T, Hofstad EF, Mårvik R, Sánchez-Margallo FM (2017) Laparoscopic pancreas surgery: image guidance solutions. In: Laparoscopic surgery. InTech, London
Schneider C, Guerrero J, Nguan C, Rohling R, Salcudean S (2011) Intra-operative “Pick-Up” ultrasound for robot assisted surgery with vessel extraction and registration: a feasibility study. In: International Conference on Information Processing in Computer-Assisted Interventions, IPCAI 2001, Lecture Notes in Computer Science, vol 6689, 122–132, Berlin, Heidelberg
Pratt P, Di Marco A, Payne C, Darzi A, Yang GZ (2012) Intraoperative ultrasound guidance for transanal endoscopic microsurgery. Med Image Comput Comput Assist Interv 15(1):463–470
Pratt P, Jaeger A, Hughes-Hallett A, Mayer E, Vale J, Darzi A, Peters T, Yang GZ (2015) Robust ultrasound probe tracking: initial clinical experiences during robot-assisted partial nephrectomy. Int J Comput Assist Radiol Surg 10(12):1905–1913
Jayarathne UL, McLeod AJ, Peters TM, Chen EC (2013) Robust intraoperative US probe tracking using a monocular endoscopic camera. In International Conference on Medical Image Computing and Computer-Assisted Intervention. Springer, Berlin, Heidelberg, p 363–370
Zhang L, Ye M, Chan PL, Yang GZ (2017) Real-time surgical tool tracking and pose estimation using a hybrid cylindrical marker. Int J Comput Assist Radiol Surg 12(6):921–930
Wang J, Kobayashi E, Sakuma I (2015) Coarse-to-fine dot array marker detection with accurate edge localization for stereo visual tracking. Biomed Signal Process Control 15:49–59
Ma L, Kiyomatsu H, Nakagawa K, Wang J, Kobayashi E, Sakuma I (2018) Accurate vessel segmentation in ultrasound images using a local-phase-based snake. Biomed Signal Process Control 43:236–243
Ma L, Nakamae K, Wang J, Kiyomatsu H, Tsukihara H, Kobayashi E, Sakuma I (2017) Image-guided laparoscopic pelvic lymph node dissection using stereo visual tracking free-hand laparoscopic ultrasound. In: The 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Jeju, Korea, p 3240–3243
Belaid A, Boukerroui D, Maingourd Y, Lerallut JF (2011) Phase-based level set segmentation of ultrasound images. IEEE Trans Inf Technol Biomed 15(1):138–147
Kass M, Witkin A, Terzopoulos D (1988) Snakes: active contour models. Int J Comput Vision 1(4):321–331
Läthén G, Jonasson J, Borga M (2010) Blood vessel segmentation using multi-scale quadrature filtering. Pattern Recogn Lett 31(8):762–767
Kiyomatsu H, Wang J, Ma L, Kiyomatsu T, Kobayashi E, Sakuma I, Watanabe T (2016) Evaluation of pelvic arteries displacement by postural changes. In: The 12th Asian Conference on Computer-Aided Surgery, Daejeon, Korea
Olson CF, Abi-Rached H (2010) Wide-baseline stereo vision for terrain mapping. Mach Vis Appl 21(5):713–725
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 26108008 and a Joint Research Project. This work was also partly supported by the JST COI Grant Number JPMJCEJPMJCE1304.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Etsuko Kobayashi and Dr. Ichiro Sakuma received grants from the Japan Society for the Promotion of Science. Dr. Lei Ma, Dr. Junchen Wang, Mr. Hidemichi Kiyomatsu, and Dr. Hiroshi Tsukihara have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, L., Wang, J., Kiyomatsu, H. et al. Surgical navigation system for laparoscopic lateral pelvic lymph node dissection in rectal cancer surgery using laparoscopic-vision-tracked ultrasonic imaging. Surg Endosc 35, 6556–6567 (2021). https://doi.org/10.1007/s00464-020-08153-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-08153-8