Abstract
Background and study aims
Natural history after endoscopic resection (ER) for gastric dysplasia is still unclear. The aim of this study was to evaluate the long-term clinical outcomes and risk factors after ER for gastric dysplasia between control and cases with synchronous or metachronous gastric neoplasm.
Methods
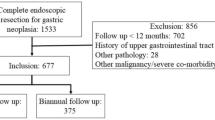
A total of 1090 patients who had undergone ER for gastric dysplasia and been followed up for at least one year from December 2002 to December 2013 were finally analyzed. Risk factors affecting the development of synchronous or metachronous neoplasm (SMN) and long-term clinical outcomes after ER for gastric dysplasia were evaluated.
Results
Synchronous and metachronous neoplasms had developed in 126 (11.6%) and 133 patients (12.2%) during the mean follow-up duration of 63.6 months, respectively. Five-year and 10-year risk of metachronous neoplasm were 9.8% and 27.2%, respectively. Median duration to the development of metachronous neoplasm was 103.1 months. While age (P < 0.001) and mucosal atrophy (P = 0.09) of index cases were associated with the development of synchronous neoplasm, age (P = 0.017), incomplete resection (P = 0.025), and intestinal metaplasia (P = 0.017) of background mucosa of index cases were significantly related to the development of metachronous neoplasm in multivariate analysis. Cumulative incidence of SMN was not significantly different among H. pylori negative, eradicated, and persistent group.
Conclusions
Age, incomplete ER, and background intestinal metaplasia of index gastric dysplasia were significantly associated with metachronous recurrence. Endoscopic surveillance for metachronous recurrence after ER for gastric dysplasia is mandatory for longer than 10 years.





Similar content being viewed by others
Abbreviations
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- EGC:
-
Early gastric cancer
- SMN:
-
Synchronous or metachronous neoplasm
- H. pylori :
-
Helicobacter pylori
- ER:
-
Endoscopic resection
- ESD:
-
Endoscopic submucosal dissection
- EMR:
-
Endoscopic mucosal resection
- APC:
-
Argon plasma coagulation
References
Correa P (1995) Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol 19:S37–43
Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ (2001) Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 345:784–789
Committee ASoP, Evans JA, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Fisher DA, Foley K, Hwang JH, Jue TL, Lightdale JR, Pasha SF, Sharaf R, Shergill AK, Cash BD, DeWitt JM (2015) The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest Endosc 82:1–8
Dixon MF (2002) Gastrointestinal epithelial neoplasia: Vienna revisited. Gut 51:130–131
Yoon SB, Park JM, Lim CH, Kim JS, Cho YK, Lee BI, Lee IS, Kim SW, Choi MG (2016) Incidence of gastric cancer after endoscopic resection of gastric adenoma. Gastrointest Endosc 83:1176–1183
Song JH, Yang SY, Lim JH, Choi JM, Kim SG (2017) The effect of helicobacter pylori eradication on the metachronous neoplasm after endoscopic resection for gastric dysplasia. Korean J Gastroenterol 70:27–32
Moon HS, Yun GY, Kim JS, Eun HS, Kang SH, Sung JK, Jeong HY, Song KS (2017) Risk factors for metachronous gastric carcinoma development after endoscopic resection of gastric dysplasia: Retrospective, single-center study. World J Gastroenterol 23:4407–4415
Chung GE, Chung SJ, Yang JI, Jin EH, Park MJ, Kim SG, Kim JS (2017) Development of metachronous tumors after endoscopic resection for gastric neoplasm according to the baseline tumor grade at a health checkup center. Korean J Gastroenterol 70:223–231
Baek DH, Kim GH, Park DY, Lee BE, Jeon HK, Lim W, Song GA (2015) Gastric epithelial dysplasia: characteristics and long-term follow-up results after endoscopic resection according to morphological categorization. BMC Gastroenterol 15:17
Japanese Gastric Cancer A (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14:101–112.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (1994) Schistosomes, liver flukes and Helicobacter pylori. International Agency for Research on Cancer, Lyon
Suerbaum S, Michetti P (2002) Helicobacter pylori infection. N Engl J Med 347:1175–1186
Ito M, Haruma K, Kamada T, Mihara M, Kim S, Kitadai Y, Sumii M, Tanaka S, Yoshihara M, Chayama K (2002) Helicobacter pylori eradication therapy improves atrophic gastritis and intestinal metaplasia: a 5-year prospective study of patients with atrophic gastritis. Aliment Pharmacol Ther 16:1449–1456
Choi IJ, Kook MC, Kim YI, Cho SJ, Lee JY, Kim CG, Park B, Nam BH (2018) Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med 378:1085–1095
Choi JM, Kim SG, Choi J, Park JY, Oh S, Yang HJ, Lim JH, Im JP, Kim JS, Jung HC (2018) Effects of Helicobacter pylori eradication for metachronous gastric cancer prevention: a randomized controlled trial. Gastrointest Endosc 88:475–485
Cho SJ, Choi IJ, Kook MC, Yoon H, Park S, Kim CG, Lee JY, Lee JH, Ryu KW, Kim YW (2013) Randomised clinical trial: the effects of Helicobacter pylori eradication on glandular atrophy and intestinal metaplasia after subtotal gastrectomy for gastric cancer. Aliment Pharmacol Ther 38:477–489
Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM, European H, Microbiota Study G, Consensus p (2017) Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 66:6–30
Chen HN, Wang Z, Li X, Zhou ZG (2016) Helicobacter pylori eradication cannot reduce the risk of gastric cancer in patients with intestinal metaplasia and dysplasia: evidence from a meta-analysis. Gastric Cancer 19:166–175
Bae SE, Jung HY, Kang J, Park YS, Baek S, Jung JH, Choi JY, Kim MY, Ahn JY, Choi KS, Kim DH, Lee JH, Choi KD, Song HJ, Lee GH, Kim JH (2014) Effect of Helicobacter pylori eradication on metachronous recurrence after endoscopic resection of gastric neoplasm. Am J Gastroenterol 109:60–67
Chon I, Choi C, Shin CM, Park YS, Kim N, Lee DH (2013) Effect of Helicobacter pylori eradication on subsequent dysplasia development after endoscopic resection of gastric dysplasia. Korean J Gastroenterol 61:307
Shin SH, Jung DH, Kim JH, Chung HS, Park JC, Shin SK, Lee SK, Lee YC (2015) Helicobacter pylori eradication prevents metachronous gastric neoplasms after endoscopic resection of gastric dysplasia. PLoS ONE 10:e0143257
Yoon H, Kim N, Shin CM, Lee HS, Kim BK, Kang GH, Kim JM, Kim JS, Lee DH, Jung HC (2016) Risk factors for metachronous gastric neoplasms in patients who underwent endoscopic resection of a gastric neoplasm. Gut Liver 10:228–236
Kim SG (2016) Treatment strategy after incomplete endoscopic resection of early gastric cancer. ClinEndosc 49:332–335
Kim TK, Kim GH, Park DY, Lee BE, Jeon TY, Kim DH, Jo HJ, Song GA (2015) Risk factors for local recurrence in patients with positive lateral resection margins after endoscopic submucosal dissection for early gastric cancer. Surg Endosc 29:2891–2898
Lee JH, Lee JH, Kim KM, Kang KJ, Min BH, Kim JJ (2015) Clinicopathological factors of multiple lateral margin involvement after endoscopic submucosal dissection for early gastric cancer. Surg Endosc 29:3460–3468
Kato M, Nishida T, Yamamoto K, Hayashi S, Kitamura S, Yabuta T, Yoshio T, Nakamura T, Komori M, Kawai N (2013) Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka University ESD study group. Gut 62:1425–1432
Abe S, Oda I, Suzuki H, Nonaka S, Yoshinaga S, Nakajima T, Sekiguchi M, Mori G, Taniguchi H, Sekine S, Katai H, Saito Y (2015) Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy 47:1113–1118
Acknowledgements
Jue Lie Kim and Sang Gyun Kim involved in analysis and interpretation of the data and drafted the article; Jue Lie Kim, Sang Gyun Kim, Ayoung Lee, Jinju Choi, Hyunsoo Chung, and Soo-Jeong Cho did critical revision of the article for important intellectual content; Sang Gyun Kim contributed to the final approval of the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
This work was supported by a Grant (NRF-2017R1D1A1B03036304) of the Basic Science Research Program through the National Research Foundation (NRF) funded by the Ministry of Education and a grant from Liver Research Institute, Seoul National University College of Medicine, Republic of Korea. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors do not have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, J.L., Kim, S.G., Lee, A. et al. Long-term natural history after endoscopic resection for gastric dysplasia. Surg Endosc 35, 5247–5255 (2021). https://doi.org/10.1007/s00464-020-08023-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-08023-3