Abstract
Background
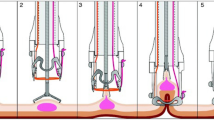
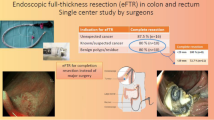
Introduction of the full-thickness resection device (FTRD) has allowed endoscopic resection of difficult lesions such as those with deep wall origin/infiltration or those located in difficult anatomic locations. The aim of this study is to assess the outcomes of the FTRD among its early users in the USA.
Methods
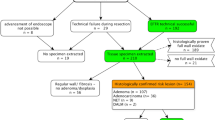
Patients who underwent endoscopic full-thickness resection (EFTR) for lower gastrointestinal tract lesions using the FTRD at 26 US tertiary care centers between 10/2017 and 12/2018 were included. Primary outcome was R0 resection rate. Secondary outcomes included rate of technical success (en bloc resection), achievement of histologic full-thickness resection (FTR), and adverse events (AE).
Results
A total of 95 patients (mean age 65.5 ± 12.6 year, 38.9% F) were included. The most common indication, for use of FTRD, was resection of difficult adenomas (non-lifting, recurrent, residual, or involving appendiceal orifice/diverticular opening) (66.3%), followed by adenocarcinomas (22.1%), and subepithelial tumors (SET) (11.6%). Lesions were located in the proximal colon (61.1%), distal colon (18.9%), or rectum (20%). Mean lesion diameter was 15.5 ± 6.4 mm and 61.1% had a prior resection attempt. The mean total procedure time was 59.7 ± 31.8 min. R0 resection was achieved in 82.7% while technical success was achieved in 84.2%. Histologically FTR was demonstrated in 88.1% of patients. There were five clinical AE (5.3%) with 2 (2.1%) requiring surgical intervention.
Conclusions
Results from this first US multicenter study suggest that EFTR with the FTRD is a technically feasible, safe, and effective technique for resecting difficult colonic lesions.




Similar content being viewed by others
Abbreviations
- AE:
-
Adverse event
- APC:
-
Argon plasma coagulation
- EFTR:
-
Endoscopic full-thickness resection
- EMR:
-
Endoscopic mucosal resection
- ESD:
-
Endoscopic submucosal dissection
- FTRD:
-
Full-thickness resection device
- HF:
-
High-frequency
- OTSC:
-
Over-the-scope clip
References
Raju GS, Lum PJ, Ross WA, Thirumurthi S, Miller E, Lynch PM, Lee JH, Bhutani MS, Shafi MA, Weston BR, Pande M, Bresalier RS, Rashid A, Mishra L, Davila ML, Stroehlein JR (2016) Outcome of EMR as an alternative to surgery in patients with complex colon polyps. Gastrointest Endosc 84:315–325
Moss A, Williams SJ, Hourigan LF, Brown G, Tam W, Singh R, Zanati S, Burgess NG, Sonson R, Byth K, Bourke MJ (2015) Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut 64:57–65
Oka S, Tanaka S, Saito Y, Iishi H, Kudo SE, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K, Hisabe T, Tsuruta O, Sano Y, Yamano H, Shimizu S, Yahagi N, Watanabe T, Nakamura H, Fujii T, Ishikawa H, Sugihara K (2015) Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol 110:697–707
Burgess NG, Bourke MJ (2016) Endoscopic resection of colorectal lesions: the narrowing divide between East and West. Dig Endosc 28:296–305
Agapov M, Dvoinikova E (2014) Factors predicting clinical outcomes of endoscopic submucosal dissection in the rectum and sigmoid colon during the learning curve. Endosc Int Open 2:E235–240
He YQ, Wang X, Li AQ, Yang L, Zhang J, Kang Q, Tang S, Jin P, Sheng JQ (2015) Factors for Endoscopic submucosal dissection in early colorectal neoplasms: a single center clinical experience in China. Clin Endosc 48:405–410
Lee SP, Kim JH, Sung IK, Lee SY, Park HS, Shim CS, Han HS (2015) Effect of submucosal fibrosis on endoscopic submucosal dissection of colorectal tumors: pathologic review of 173 cases. J Gastroenterol Hepatol 30:872–878
Mizushima T, Kato M, Iwanaga I, Sato F, Kubo K, Ehira N, Uebayashi M, Ono S, Nakagawa M, Mabe K, Shimizu Y, Sakamoto N (2015) Technical difficulty according to location, and risk factors for perforation, in endoscopic submucosal dissection of colorectal tumors. Surg Endosc 29:133–139
Hong SN, Byeon JS, Lee BI, Yang DH, Kim J, Cho KB, Cho JW, Jang HJ, Jeon SW, Jung SA, Chang DK (2016) Prediction model and risk score for perforation in patients undergoing colorectal endoscopic submucosal dissection. Gastrointest Endosc 84:98–108
Fahndrich M, Sandmann M (2015) Endoscopic full-thickness resection for gastrointestinal lesions using the over-the-scope clip system: a case series. Endoscopy 47:76–79
Monkemuller K, Peter S, Toshniwal J, Popa D, Zabielski M, Stahl RD, Ramesh J, Wilcox CM (2014) Multipurpose use of the 'bear claw' (over-the-scope-clip system) to treat endoluminal gastrointestinal disorders. Dig Endosc 26:350–357
Schmidt A, Bauerfeind P, Gubler C, Damm M, Bauder M, Caca K (2015) Endoscopic full-thickness resection in the colorectum with a novel over-the-scope device: first experience. Endoscopy 47:719–725
Andrisani G, Pizzicannella M, Martino M, Rea R, Pandolfi M, Taffon C, Caricato M, Coppola R, Crescenzi A, Costamagna G, Di Matteo FM (2017) Endoscopic full-thickness resection of superficial colorectal neoplasms using a new over-the-scope clip system: a single-centre study. Dig Liver Dis 49:1009–1013
Valli PV, Mertens J, Bauerfeind P (2018) Safe and successful resection of difficult GI lesions using a novel single-step full-thickness resection device (FTRD((R))). Surg Endosc 32:289–299
Schmidt A, Beyna T, Schumacher B, Meining A, Richter-Schrag HJ, Messmann H, Neuhaus H, Albers D, Birk M, Thimme R, Probst A, Faehndrich M, Frieling T, Goetz M, Riecken B, Caca K (2018) Colonoscopic full-thickness resection using an over-the-scope device: a prospective multicentre study in various indications. Gut 67:1280–1289
Schmidt A, Damm M, Caca K (2014) Endoscopic full-thickness resection using a novel over-the-scope device. Gastroenterology 147:740–742.e742
Richter-Schrag HJ, Walker C, Thimme R, Fischer A (2016) Full thickness resection device (FTRD). Experience and outcome for benign neoplasms of the rectum and colon. Chirurg 87:316–325
Sarker S, Gutierrez JP, Council L, Brazelton JD, Kyanam Kabir Baig KR, Monkemuller K (2014) Over-the-scope clip-assisted method for resection of full-thickness submucosal lesions of the gastrointestinal tract. Endoscopy 46:758–761
Lagoussis P, Soriani P, Tontini GE, Neumann H, Pastorelli L, de Nucci G, Vecchi M (2016) Over-the-scope clip-assisted endoscopic full-thickness resection after incomplete resection of rectal adenocarcinoma. Endoscopy 48(Suppl 1):E59–E60
Soriani P, Tontini GE, Neumann H, de Nucci G, De Toma D, Bruni B, Vavassori S, Pastorelli L, Vecchi M, Lagoussis P (2017) Endoscopic full-thickness resection for T1 early rectal cancer: a case series and video report. Endosc Int Open 5:E1081–1086
Aepli P, Criblez D, Baumeler S, Borovicka J, Frei R (2018) Endoscopic full thickness resection (EFTR) of colorectal neoplasms with the Full Thickness Resection Device (FTRD): Clinical experience from two tertiary referral centers in Switzerland. United Eur Gastroenterol J 6:463–470
Quirke P, Risio M, Lambert R, von Karsa L, Vieth M (2011) Quality assurance in pathology in colorectal cancer screening and diagnosis-European recommendations. Virchows Arch 458:1–19
Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A Jr, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ (2010) A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc 71:446–454
Aslanian HR, Sethi A, Bhutani MS, Goodman AJ, Krishnan K, Lichtenstein DR, Melson J, Navaneethan U, Pannala R, Parsi MA, Schulman AR, Sullivan SA, Thosani N, Trikudanathan G, Trindade AJ, Watson RR, Maple JT (2019) ASGE guideline for endoscopic full-thickness resection and submucosal tunnel endoscopic resection. VideoGIE 4:343–350
Isomoto H, Nishiyama H, Yamaguchi N, Fukuda E, Ishii H, Ikeda K, Ohnita K, Nakao K, Kohno S, Shikuwa S (2009) Clinicopathological factors associated with clinical outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy 41:679–683
Hori K, Uraoka T, Harada K, Higashi R, Kawahara Y, Okada H, Ramberan H, Yahagi N, Yamamoto K (2014) Predictive factors for technically difficult endoscopic submucosal dissection in the colorectum. Endoscopy 46:862–870
Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, Fukuzawa M, Kobayashi N, Nasu J, Michida T, Yoshida S, Ikehara H, Otake Y, Nakajima T, Matsuda T, Saito D (2010) A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 72:1217–1225
Bucalau AM, Lemmers A, Arvanitakis M, Blero D, Neuhaus H (2018) Endoscopic Full-thickness resection of a colonic lateral spreading tumor. Dig Dis 36:252–256
Bronzwaer ME, Bastiaansen BA, Koens L, Dekker E, Fockens P (2018) Endoscopic full-thickness resection of polyps involving the appendiceal orifice: a prospective observational case study. Endosc Int Open 6:E1112–1119
Ahlenstiel G, Hourigan LF, Brown G, Zanati S, Williams SJ, Singh R, Moss A, Sonson R, Bourke MJ (2014) Actual endoscopic versus predicted surgical mortality for treatment of advanced mucosal neoplasia of the colon. Gastrointest Endosc 80:668–676
Kuellmer A, Mueller J, Caca K, Aepli P, Albers D, Schumacher B, Glitsch A, Schafer C, Wallstabe I, Hofmann C, Erhardt A, Meier B, Bettinger D, Thimme R, Schmidt A (2019) Endoscopic full-thickness resection for early colorectal cancer. Gastrointest Endosc 89:1180–1189.e1181
Schreiner P, Valli P, Marques Maggio E, Bauerfeind P (2018) Simultaneous endoscopic full-thickness resection of two synchronous colonic granular cell tumours. BMJ Case Rep. https://doi.org/10.1136/bcr-2017-222223
Author information
Authors and Affiliations
Contributions
MK, YI, and KV were involved in the study planning, interpretation of the data, and development of the manuscript. DD, GI, JT, and TA were involved in critically revising the manuscript for important intellectual content. All remaining authors involved in performing the procedure and data extraction. All authors approved the final submitted draft of this manuscript.
Corresponding author
Ethics declarations
Disclosures
Dr. Khashab is consultant for Boston Scientific, Medtronic, and Olympus. Dr. Ian Grimm is a consultant for Boston Scientific. Dr. Irani is a consultant for Boston Scientific. Dr. Kumbhari is a consultant for ReShape Life Sciences, Apollo Endosurgery, Medtronic, and Boston Scientific. Dr. Nikhil is a consultant for Apollo Endosurgery, Boston scientific, and Olympus. Dr. Amateau is a consultant for Merit Endoscopy, Boston Scientific, US Endoscopy, and Neurotronic and the recipient of research support from Cook Medical. Dr. Smallfield has research funding from CSA medical and C2 therapeutics. Dr. Aadam AA is a consultant for Boston Scientific. Dr. Diehl Md Consultant for Boston Scientific, Olympus, Pentax, Cook Medical, Merit, ConMed, US Endoscopy, Medtronic, Lumendi. Dr. Chang consultant for Apollo, Boston Scientific, Cook, Covidien, Erbe, Endogastric Solutions, Mauna Kea Mederi, Medtronic, Olympus, Ovesco, Pentax, Torax. Dr. Samarasena has educational grant from Cook and consultant for Mauna Kea, Medtronic, Olympus, Pentax, US Endoscopy. Dr. Al-Haddad received research and teaching support from Boston Scientific. Dr. Pohl received grants from Boston Scientific, US Endoscopy, and Aries/Cosmo Pharamceuticals. Dr. Templeton is a consultant for Boston Scientific and Medtronic. Dr. Ginsberg is a consultant for Olympus Inc. and Boston Scientific. Dr. Fukami is consultant for Boston Scientific and Olympus. Dr. Sharaiha is consultant for Boston Scientific, Olympus, Apollo, and Medtronic. Dr. Ichkhanian, Dr. Vosoughi, Dr. James, Dr. Hajifathalian, Dr. Tokar, Dr. Lee, Dr. Mizrahi, Dr. Barawi, Dr. Friedland, Dr. Korc, Dr. Kowalski, Dr. Novikov, Dr. Oza, Dr. Panuu, Dr. Lajin, Dr. Kumta, Dr. Tang, Dr. Naga, and Dr. Brewer have no conflicts of interest or financial ties to disclose
Ethical approval
IRB approval for this study across the multiple centers performed in accordance with the principles of the Declaration of Helsinki. The formal informed consent was waived.
Informed consent
The formal informed consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 91407 kb)
Rights and permissions
About this article
Cite this article
Ichkhanian, Y., Vosoughi, K., Diehl, D.L. et al. A large multicenter cohort on the use of full-thickness resection device for difficult colonic lesions. Surg Endosc 35, 1296–1306 (2021). https://doi.org/10.1007/s00464-020-07504-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-07504-9