Abstract
Introduction
Probe-based confocal laser endomicroscopy (pCLE) is an innovative technique providing real-time, in vivo optical biopsies. A previous ex vivo phase of the study (PERSEE) allowed identifying accurate pCLE criteria for the diagnosis of hepatic and peritoneal surgical specimens. This study aimed at evaluating the pCLE role for in vivo intra-abdominal tissue characterization during digestive cancer surgical procedures.
Methods
Between October 2014 and July 2015, consecutive patients diagnosed with digestive cancers and scheduled for a surgical resection or an exploratory laparoscopy were prospectively enrolled. Endomicroscopic images were acquired using a motorized Confocal Miniprobe™ with a bending distal tip providing easy access to abdominal organs. It was connected to an endomicroscopy system that allowed near-infrared illumination (at a wavelength of 785 nm) in conjunction with indocyanine green for contrast agent. A live audiovisual transmission was established between the surgeon and the pathologist for real-time interpretation of optical biopsies. Intraoperative pCLE performance for the diagnosis of suspicious nodules was assessed using corresponding surgical histopathology as reference standard.
Results
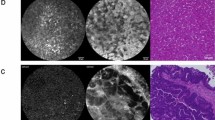
21 consecutive patients were successfully enrolled. Live audiovisual transmission between the surgeon and the pathologist was successfully established in all cases. 62 pCLE sequences were acquired from different tissues [peritoneum (n = 27), liver (n = 21), lymph node (n = 4), diaphragm (n = 3), colon (n = 3), stomach (n = 2), and adrenal gland (n = 2)]. Malignant tissues were identified by fluorescently enhanced irregular cancerous tubes contrasting with dark glandular lumen and extracellular matrix. pCLE sensitivities and specificities were 67% and 100%, and 38% and 100% for peritoneal and hepatic carcinogenesis, respectively. One benign incident was reported during the trial with no patient consequence.
Conclusions
Real-time intraoperative pCLE with near-infrared illumination is feasible and safe, provides additional information in terms of tissue characterization, and, in combination with telepathology, allows interactive collaboration between the surgeon and the pathologist during surgical procedures.
Trial registration clinicaltrials.gov Identifier: NCT02312167.




Similar content being viewed by others
References
Hoch G, Croise-Laurent V, Germain A, Brunaud L, Bresler L, Ayav A (2015) Is intraoperative ultrasound still useful for the detection of colorectal cancer liver metastases? HPB 17:514–519. https://doi.org/10.1111/hpb.12393
Dromain C, Leboulleux S, Auperin A, Goere D, Malka D, Lumbroso J, Schumberger M, Sigal R, Elias D (2008) Staging of peritoneal carcinomatosis: enhanced CT vs. PET/CT. Abdom Imaging 33:87–93. https://doi.org/10.1007/s00261-007-9211-7
Jarnagin WR, Conlon K, Bodniewicz J, Dougherty E, DeMatteo RP, Blumgart LH, Fong Y (2001) A clinical scoring system predicts the yield of diagnostic laparoscopy in patients with potentially resectable hepatic colorectal metastases. Cancer 91:1121–1128
Grobmyer SR, Fong Y, D’Angelica M, Dematteo RP, Blumgart LH, Jarnagin WR (2004) Diagnostic laparoscopy prior to planned hepatic resection for colorectal metastases. Arch Surg 139:1326–1330. https://doi.org/10.1001/archsurg.139.12.1326
Oberschmid B, Dietrich A, Wittekind C (2012) Frozen sections diagnostics in visceral surgery. Stomach and intestines. Pathol 33:407–412. https://doi.org/10.1007/s00292-012-1601-0
Sharma P, Meining AR, Coron E, Lightdale CJ, Wolfsen HC, Bansal A, Bajbouj M, Galmiche J-P, Abrams JA, Rastogi A, Gupta N, Michalek JE, Lauwers GY, Wallace MB (2011) Real-time increased detection of neoplastic tissue in Barrett’s esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. Gastrointest Endosc 74:465–472. https://doi.org/10.1016/j.gie.2011.04.004
Gaddam S, Mathur SC, Singh M, Arora J, Wani SB, Gupta N, Overhiser A, Rastogi A, Singh V, Desai N, Hall SB, Bansal A, Sharma P (2011) Novel probe-based confocal laser endomicroscopy criteria and interobserver agreement for the detection of dysplasia in barrett’s esophagus. Am J Gastroenterol 106:1961–1969. https://doi.org/10.1038/ajg.2011.294
Neumann H, Kiesslich R (2013) Endomicroscopy and endocytoscopy in IBD. Gastrointest Endosc Clin N Am 23:695–705. https://doi.org/10.1016/j.giec.2013.03.006
Chang TC, Liu J-J, Hsiao ST, Pan Y, Mach KE, Leppert JT, McKenney JK, Rouse RV, Liao JC (2013) Interobserver agreement of confocal laser endomicroscopy for bladder cancer. J Endourol 27:598–603. https://doi.org/10.1089/end.2012.0549
Yserbyt J, Dooms C, Ninane V, Decramer M, Verleden G (2013) Perspectives using probe-based confocal laser endomicroscopy of the respiratory tract. Swiss Med Wkly. https://doi.org/10.4414/smw.2013.13764
Napoléon B, Lemaistre A-I, Pujol B, Caillol F, Lucidarme D, Bourdariat R, Morellon-Mialhe B, Fumex F, Lefort C, Lepilliez V, Palazzo L, Monges G, Filoche B, Giovannini M (2014) A novel approach to the diagnosis of pancreatic serous cystadenoma: needle-based confocal laser endomicroscopy. Endoscopy 47:26–32. https://doi.org/10.1055/s-0034-1390693
Slivka A, Gan I, Jamidar P, Costamagna G, Cesaro P, Giovannini M, Caillol F, Kahaleh M (2015) Validation of the diagnostic accuracy of probe-based confocal laser endomicroscopy for the characterization of indeterminate biliary strictures: results of a prospective multicenter international study. Gastrointest Endosc 81:282–290. https://doi.org/10.1016/j.gie.2014.10.009
Pierangelo A, Fuks D, Benali A, Validire P, Gayet B (2017) Diagnostic accuracy of confocal laser endomicroscopy for the ex vivo characterization of peritoneal nodules during laparoscopic surgery. Surg Endosc 31:1974–1981. https://doi.org/10.1007/s00464-016-5172-7
Pierangelo A, Fuks D, Validire P, Benali A, Gayet B (2017) Diagnostic accuracy of confocal laser endomicroscopy for the characterization of liver nodules. Eur J Gastroenterol Hepatol 29:42–47. https://doi.org/10.1097/MEG.0000000000000741
Weinstein RS, Bloom KJ, Rozek LS (1987) Telepathology and the networking of pathology diagnostic services. Arch Pathol Lab Med 111:646–652
Kayser K, Drlicek M, Rahn W (1993) Aids of telepathology in intra-operative histomorphological tumor diagnosis and classification. Vivo Athens Greece 7:395–398
Kayser K, Fritz P, Drlicek M (1995) Aspects of telepathology in routinary diagnostic work with specific emphasis on ISDN. Arch Anat Cytol Pathol 43:216–218
Nordrum I, Engum B, Rinde E, Finseth A, Ericsson H, Kearney M, Stalsberg H, Eide TJ (1991) Remote frozen section service: a telepathology project in northern Norway. Hum Pathol 22:514–518
Vitkovski T, Bhuiya T, Esposito M (2015) Utility of telepathology as a consultation tool between an off-site surgical pathology suite and affiliated hospitals in the frozen section diagnosis of lung neoplasms. J Pathol Inform 6:55. https://doi.org/10.4103/2153-3539.168515
Goetz M, Kiesslich R, Dienes H-P, Drebber U, Murr E, Hoffman A, Kanzler S, Galle P, Delaney P, Neurath M (2008) In vivo confocal laser endomicroscopy of the human liver: a novel method for assessing liver microarchitecture in real time. Endoscopy 40:554–562. https://doi.org/10.1055/s-2008-1077296
Goetz M, Deris I, Vieth M, Murr E, Hoffman A, Delaney P, Galle PR, Neurath MF, Kiesslich R (2010) Near-infrared confocal imaging during mini-laparoscopy: a novel rigid endomicroscope with increased imaging plane depth. J Hepatol 53:84–90. https://doi.org/10.1016/j.jhep.2010.01.039
Lopez A, Zlatev DV, Mach KE, Bui D, Liu J-J, Rouse RV, Harris T, Leppert JT, Liao JC (2016) Intraoperative optical biopsy during robotic assisted radical prostatectomy using confocal endomicroscopy. J Urol 195:1110–1117. https://doi.org/10.1016/j.juro.2015.10.182
Ishizawa T, Fukushima N, Shibahara J, Masuda K, Tamura S, Aoki T, Hasegawa K, Beck Y, Fukayama M, Kokudo N (2009) Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 115:2491–2504. https://doi.org/10.1002/cncr.24291
Ferrero A, Langella S, Giuliante F, Viganò L, Vellone M, Zimmitti G, Ardito F, Nuzzo G, Capussotti L (2013) Intraoperative liver ultrasound still affects surgical strategy for patients with colorectal metastases in the modern era. World J Surg 37:2655–2663. https://doi.org/10.1007/s00268-013-2183-6
Tamandl D, Herberger B, Gruenberger B, Schoppmann SF, Puhalla H, Schindl M, Schima W, Jakesz R, Gruenberger T (2008) Adequate preoperative staging rarely leads to a change of intraoperative strategy in patients undergoing surgery for colorectal cancer liver metastases. Surgery 143:648–657. https://doi.org/10.1016/j.surg.2007.11.020
Niekel MC, Bipat S, Stoker J (2010) Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology 257:674–684. https://doi.org/10.1148/radiol.10100729
Wagnetz U, Atri M, Massey C, Wei AC, Metser U (2011) Intraoperative ultrasound of the liver in primary and secondary hepatic malignancies: comparison with preoperative 1.5-T MRI and 64-MDCT. AJR Am J Roentgenol 196:562–568. https://doi.org/10.2214/AJR.10.4729
Napoleon B, Lemaistre A-I, Pujol B, Caillol F, Lucidarme D, Bourdariat R, Morellon-Mialhe B, Fumex F, Lefort C, Lepilliez V, Palazzo L, Monges G, Poizat F, Giovannini M (2016) In vivo characterization of pancreatic cystic lesions by needle-based confocal laser endomicroscopy (nCLE): proposition of a comprehensive nCLE classification confirmed by an external retrospective evaluation. Surg Endosc 30:2603–2612. https://doi.org/10.1007/s00464-015-4510-5
Cherrick GR, Stein SW, Leevy CM, Davidson CS (1960) Indocyanine green: observations on its physical properties, plasma decay, and hepatic extraction. J Clin Invest 39:592–600. https://doi.org/10.1172/JCI104072
Gotoh K, Yamada T, Ishikawa O, Takahashi H, Eguchi H, Yano M, Ohigashi H, Tomita Y, Miyamoto Y, Imaoka S (2009) A novel image-guided surgery of hepatocellular carcinoma by indocyanine green fluorescence imaging navigation. J Surg Oncol 100:75–79. https://doi.org/10.1002/jso.21272
Liberale G, Bourgeois P, Larsimont D, Moreau M, Donckier V, Ishizawa T (2017) Indocyanine green fluorescence-guided surgery after IV injection in metastatic colorectal cancer: a systematic review. Eur J Surg Oncol 43:1656–1667. https://doi.org/10.1016/j.ejso.2017.04.015
Shimada S, Ohtsubo S, Ogasawara K, Kusano M (2015) Macro- and microscopic findings of ICG fluorescence in liver tumors. World J Surg Oncol 13:198. https://doi.org/10.1186/s12957-015-0615-5
Funding
The study was funded by BPI France: #I0911038W.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Prof. Brice Gayet is a consultant for Mauna Kea Technologies. Dr. Pierangelo, Prof. Fuks, Dr. Validire, and Prof. Gayet have received funding from Mauna Kea Technologies to support congress registration and travel fees. Guillaume Trebuchet and Aline Criton are employees of Mauna Kea Technologies. Dr. Benali and Dr. Lefevre have no conflicts of interest or financial ties to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fuks, D., Pierangelo, A., Validire, P. et al. Intraoperative confocal laser endomicroscopy for real-time in vivo tissue characterization during surgical procedures. Surg Endosc 33, 1544–1552 (2019). https://doi.org/10.1007/s00464-018-6442-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6442-3