Abstract
Background
Fluorescence technology with indocyanine green (ICG) provides a real-time assessment of intestinal perfusion. However, a subjective evaluation of fluorescence intensity based on the surgeon’s visual judgement is a major limitation. This study evaluated the quantitative assessment of ICG fluorescence imaging in determining the transection line of the proximal colon during laparoscopic colorectal surgery.
Methods
This is a retrospective analysis of a prospectively maintained database of 112 patients who underwent laparoscopic surgery for left-sided colorectal cancers. After distal transection of the bowel, the specimen was extracted extracorporeally and then the proximal colon was divided within the well-perfused area based on the ICG fluorescence imaging. We evaluated whether quantitative assessment of intestinal perfusion by measuring ICG intensity could predict postoperative outcomes: F max, T max, T 1/2, and Slope were calculated.
Results
Anastomotic leakage (AL) occurred in 5 cases (4.5%). Based on the fluorescence imaging, the surgical team opted for further proximal change of the transection line up to an “adequate” fluorescent portion in 18 cases (16.1%). Among the 18 patients, AL occurred in 4 patients (4/18: 22.2%), whereas it occurred in only 1 case (1/94: 1.0%) in the good perfusion patients who did not need proximal change of the transection line. The F max of the AL group was less than 52.0 in all 5 cases (5/5), whereas that of the non-AL group was in only 8 cases (8/107): with an F max cutoff value of 52.0, the sensitivity and specificity for the prediction of AL were 100 and 92.5%, respectively. Regarding postoperative bowel movement recovery, the T max of the early flatus group or early defecation group was significantly lower than that of the late flatus group or late defecation group, respectively.
Conclusions
ICG fluorescence imaging is useful for assessing anastomotic perfusion in colorectal surgery, which can result in more precise operative decisions tailored for an individual patient.



Similar content being viewed by others
References
Kingham TP, Pachter HL (2009) Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg 208:269–278
Branagan G, Finnis D; Wessex Colorectal Cancer Audit Working Group (2005) Prognosis after anastomotic leakage in colorectal surgery. Dis Colon Rectum 48:1021–1026
Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P (2011) Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg 253:890–899
Kang CY, Halabi WJ, Chaudhry OO, Nguyen V, Pigazzi A, Carmichael JC, Mills S, Stamos MJ (2013) Risk factors for anastomotic leakage after anterior resection for rectal cancer. JAMA Surg 148:65–71
Qu H, Liu Y, Bi DS (2015) Clinical risk factors for anastomotic leakage after laparoscopic anterior resection for rectal cancer: a systematic review and meta-analysis. Surg Endosc 29:3608–3617
Shiomi A, Ito M, Maeda K, Kinugasa Y, Ota M, Yamaue H, Shiozawa M, Horie H, Kuriu Y, Saito N (2015) Effects of a diverting stoma on symptomatic anastomotic leakage after low anterior resection for rectal cancer: a propensity score matching analysis of 1,014 consecutive patients. J Am Coll Surg 220:186–194
Matsubara N, Miyata H, Gotoh M, Tomita N, Baba H, Kimura W, Nakagoe T, Simada M, Kitagawa Y, Sugihara K, Mori M (2014) Mortality after common rectal surgery in Japan: a study on low anterior resection from a newly established nationwide large-scale clinical database. Dis Colon Rectum 57:1075–1081
Vignali A, Gianotti L, Braga M, Radaelli G, Malvezzi L, Di Carlo V (2000) Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Dis Colon Rectum 43:76–82
Sheridan WG, Lowndes RH, Young HL (1987) Tissue oxygen tension as a predictor of colonic anastomotic healing. Dis Colon Rectum 30:867–871
Kologlu M, Yorganci K, Renda N, Sayek I (2000) Effect of local and remote ischemia-reperfusion injury on healing of colonic anastomoses. Surgery 128:99–104
Kawada K, Sakai Y (2016) Preoperative, intraoperative and postoperative risk factors for anastomotic leakage after laparoscopic low anterior resection with double stapling technique anastomosis. World J Gastroenterol 22:5718–5727
Nakayama S, Hasegawa S, Nagayama S, Kato S, Hida K, Tanaka E, Itami A, Kubo H, Sakai Y (2011) The importance of precompression time for secure stapling with a linear stapler. Surg Endosc 25:2382–2386
Nakayama S, Hasegawa S, Hida K, Kawada K, Sakai Y (2015) Obtaining secure stapling of a double stapling anastomosis. J Surg Res 193:652–657
Hasegawa S, Nakayama S, Hida K, Kawada K, Sakai Y (2015) Effect of tri-staple™ technology and slow firing on secure stapling using an endoscopic linear stapler. Dig Surg 32:353–360
Kawada K, Hasegawa S, Hida K, Hirai K, Okoshi K, Nomura A, Kawamura J, Nagayama S, Sakai Y (2014) Risk factors for anastomotic leakage after laparoscopic low anterior resection with DST anastomosis. Surg Endosc 28:2988–2995
Karliczek A, Harlaar NJ, Zeebregts CJ, Wiggers T, Baas PC, van Dam GM (2009) Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int J Colorectal Dis 24:569–576
Kudszus S, Roesel C, Schachtrupp A, Hoer JJ (2010) Intraoperative laser fluorescence angiography in colorectal surgery: a noninvasive analysis to reduce the rate of anastomotic leakage. Langenbecks Arch Surg 395:1025–1030
Jafari MD, Lee KH, Halabi WJ, Mills SD, Carmichael JC, Stamos MJ, Pigazzi A (2013) The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg Endosc 27:3003–3008
Hellan M, Spinoglio G, Pigazzi A, Lagares-Garcia JA (2014) The influence of fluorescence imaging on the location of bowel transection during robotic left-sided colorectal surgery. Surg Endosc 28:1695–1702
Jafari MD, Wexner SD, Martz JE, McLemore EC, Margolin DA, Sherwinter DA, Lee SW, Senagore AJ, Phelan MJ, Stamos MJ (2015) Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multi-institutional study. J Am Coll Surg 220:82–92
Degett TH, Andersen HS, Gögenur I (2016) Indocyanine green fluorescence angiography for intraoperative assessment of gastrointestinal anastomotic perfusion: a systematic review of clinical trials. Langenbecks Arch Surg 401:767–775
Kawada K, Hasegawa S, Wada T, Takahashi R, Hisamori S, Hida K, Sakai Y (2016) Evaluation of intestinal perfusion by ICG fluorescence imaging in laparoscopic colorectal surgery with DST anastomosis. Surg Endosc :1–9
Sakai Y, Kitano S (2015) Practice guidelines on endoscopic surgery for qualified surgeons by the endoscopic surgical skill qualification system. Asian J Endosc Surg 8:103–113
Hasegawa S, Nagayama S, Nomura A, Kawamura J, Sakai Y (2008) Multimedia article. Autonomic nerve-preserving total mesorectal excision in the laparoscopic era. Dis Colon Rectum 51:1279–1282
Kuroyanagi H, Oya M, Ueno M, Fujimoto Y, Yamaguchi T, Muto T (2008) Standardized technique of laparoscopic intracorporeal rectal transection and anastomosis for low anterior resection. Surg Endosc 22:557–561
Araki J, Nishizawa Y, Nakamura T, Sato T, Naito M, Fujii S, Mihara M, Koshima I (2012) The development of a canine anorectal autotransplantation model based on blood supply: a preliminary case report. PLoS ONE 7:e44310
Kawaguchi Y, Ishizawa T, Miyata Y, Yamashita S, Masuda K, Satou S, Tamura S, Kaneko J, Sakamoto Y, Aoki T, Hasegawa K, Sugawara Y, Kokudo N (2013) Portal uptake function in veno-occlusive regions evaluated by real-time fluorescent imaging using indocyanine green. J Hepatol 58:247–253
Terasaki H, Inoue Y, Sugano N, Jibiki M, Kudo T, Lepäntalo M, Venermo M (2013) A quantitative method for evaluating local perfusion using indocyanine green fluorescence imaging. Ann Vasc Surg 27:1154–1161
Igari K, Kudo T, Uchiyama H, Toyofuku T, Inoue Y (2014) Intraarterial injection of indocyanine green for evaluation of peripheral blood circulation in patients with peripheral arterial disease. Ann Vasc Surg 28:1280–1285
Kawaguchi Y, Tanaka N, Nagai M, Nomura Y, Fuks D, Gayet B, Kokudo N (2015) Usefulness of intraoperative real-time tissue elastography during laparoscopic hepatectomy. J Am Coll Surg 221:e103-e111
Kamiya K, Unno N, Miyazaki S, Sano M, Kikuchi H, Hiramatsu Y, Ohta M, Yamatodani T, Mineta H, Konno H (2015) Quantitative assessment of the free jejunal graft perfusion. J Surg Res 194:394–399
Venermo M, Settembre N, Albäck A, Vikatmaa P, Aho PS, Lepäntalo M, Inoue Y, Terasaki H (2016) Pilot assessment of the repeatability of indocyanine green fluorescence imaging and correlation with traditional foot perfusion assessments. Eur J Vasc Endovasc Surg 52:527–533
Ambrosetti P, Robert J, Mathey P, Rohner A (1994) Left-sided colon and colorectal anastomoses: Doppler ultrasound as an aid to assess bowel vascularization. A prospective evaluation of 200 consecutive elective cases. Int J Colorectal Dis 9:211–214
Boyle NH, Manifold D, Jordan MH, Mason RC (2000) Intraoperative assessment of colonic perfusion using scanning laser Doppler flowmetry during colonic resection. J Am Coll Surg 191:504–510
Karliczek A, Benaron DA, Baas PC, Zeebregts CJ, Wiggers T, van Dam GM (2010) Intraoperative assessment of microperfusion with visible light spectroscopy for prediction of anastomotic leakage in colorectal anastomoses. Colorectal Dis 12:1018–1025
Still J, Law E, Dawson J, Bracci S, Island T, Holtz J (1999) Evaluation of the circulation of reconstructive flaps using laser-induced fluorescence of indocyanine green. Ann Plast Surg 42:266–274
Waseda K, Ako J, Hasegawa T, Shimada Y, Ikeno F, Ishikawa T, Demura Y, Hatada K, Yock PG, Honda Y, Fitzgerald PJ, Takahashi M (2009) Intraoperative fluorescence imaging system for on-site assessment of off-pump coronary artery bypass graft. JACC Cardiovasc Imaging 2:604–612
Yukaya T, Saeki H, Kasagi Y, Nakashima Y, Ando K, Imamura Y, Ohgaki K, Oki E, Morita M, Maehara Y (2015) Indocyanine green fluorescence angiography for quantitative evaluation of gastric tube perfusion in patients undergoing esophagectomy. J Am Coll Surg 221:e37–e42
Kin C, Vo H, Welton L, Welton M (2015) Equivocal effect of intraoperative fluorescence angiography on colorectal anastomotic leaks. Dis Colon Rectum 58:582–587
Boni L, Fingerhut A, Marzorati A, Rausei S, Dionigi G, Cassinotti E (2016) Indocyanine green fluorescence angiography during laparoscopic low anterior resection: results of a case-matched study. Surg Endosc:1–5
Matsui A, Winer JH, Laurence RG, Frangioni JV (2011) Predicting the survival of experimental ischaemic small bowel using intraoperative near-infrared fluorescence angiography. Br J Surg 98:1725–1734
Diana M, Noll E, Diemunsch P, Dallemagne B, Benahmed MA, Agnus V, Soler L, Barry B, Namer IJ, Demartines N, Charles AL, Geny B, Marescaux J (2014) Enhanced-reality video fluorescence: a real-time assessment of intestinal viability. Ann Surg 259:700–707
Acknowledgements
The authors thank medical staffs and residents of Kyoto University Hospital gastrointestinal surgery for their participation in this study. We could not have completed the study without their diligence and support. We also thank Mr. Takahiro Shikayama (Hamamatsu Photonics K.K.) for technical assistance for the analysis using ROIs. We would like to thank Editage (http://www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Drs. Toshiaki Wada, Kenji Kawada, Ryo Takahashi, Mami Yoshitomi, Koya Hida, Suguru Hasegawa, and Yoshiharu Sakai have no conflicts of interest or financial ties to disclose.
Additional information
T. Wada and K. Kawada contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.

464_2017_5475_MOESM1_ESM.jpg
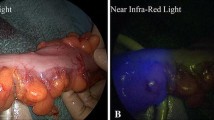
Supplementary Figure 1. A) Bowel perfusion under white/visible light. Forceps was placed at the transection line. B) Bowel perfusion under ICG fluorescence imaging. Three regions of interest (ROIs) were labeled as followed: Point 1 (mesenteric side of the transection line), Point 2 (midpoint of the transection line) and Point 3 (antimesenteric side of the transection line). C) Time curve of ICG fluorescence intensity. Point 1 is a red curve, Point 2 is a blue curve, and Point 3 is a green curve. (JPG 480 KB)
Rights and permissions
About this article
Cite this article
Wada, T., Kawada, K., Takahashi, R. et al. ICG fluorescence imaging for quantitative evaluation of colonic perfusion in laparoscopic colorectal surgery. Surg Endosc 31, 4184–4193 (2017). https://doi.org/10.1007/s00464-017-5475-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5475-3