Abstract
Background
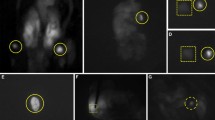
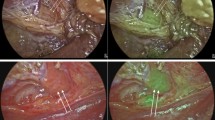
Parathyroid gland (PG) identification during thyroid and parathyroid surgery is challenging. Accidental parathyroidectomy increases the rate of postoperative hypocalcaemia. Recently, autofluorescence with near infrared light (NIRL) has been described for PG visualization. The aim of this study is to analyze the increased rate of visualization of PGs with the use of NIRL compared to white light (WL).
Materials and methods
All patients undergoing thyroid and parathyroid surgery were included in this study. PGs were identified with both NIRL and WL by experienced head and neck surgeons. The number of PGs identified with NIRL and WL were compared. The identification of PGs was correlated to age, sex, and histopathological diagnosis.
Results
Seventy-four patients were included in the study. The mean age was 48.4 (SD ±13.5) years old. Mean PG fluorescence intensity (47.60) was significantly higher compared to the thyroid gland (22.32) and background (9.27) (p < 0.0001). The mean number of PGs identified with NIRL and WL were 3.7 and 2.5 PG, respectively (p < 0.001). The difference in the number of PGs identified with NIRL and WL and fluorescence intensity was not related to age, sex, or histopathological diagnosis, with the exception of the diagnosis of thyroiditis, in which there was a significant increase in the number of PGs visualized with NIRL (p = 0.026).
Conclusion
The use of NIRL for PG visualization significantly increased the number of PGs identified during thyroid and parathyroid surgery, and the differences in fluorescent intensity among PGs, thyroid glands, and background were not affected by age, sex, and histopathological diagnosis.



Similar content being viewed by others
References
McWade MA, Paras C, White LM, Phay JE, Mahadevan-Jansen A, Broome JT (2013) A novel optical approach to intraoperative detection of parathyroid glands. Surgery 154:1371–1377
Gu J, Wang J, Nie X, Wang W, Shang J (2015) Potential role for carbon nanoparticles identification and preservation in situ of parathyroid glands during total thyroidectomy and central compartment node dissection. Int J Clin Exp Med 8:9640–9648
Okada M, Tominaga Y, Yamamoto T, Hiramitsu T, Narumi S, Watarai Y (2016) Location frequency of missed parathyroid glands after parathyroidectomy in patients with persistent or recurrent secondary hyperparathyroidism. World J Surg 40:595–599
Dumitras M, Strambu V, Radu PA, Radu PA, Iorga C, Bengulescu I, Popa F (2015) Technical factors involved in parathyroid surgery. Chirurgia 110: 425.
Sound S, Okoh A, Yigitbas H, Yazici P, Berber E (2015) Utility of indocyanine green fluorescence imaging for intraoperative localization in reoperative parathyroid surgery. Surg Innov. doi:10.1177/1553350615613450
McWade MA, Sanders ME, Broome JT, Solórzano CC, Mahadevan-Jansen A (2016) Establishing the clinical utility of autofluorescence spectroscopy for parathyroid detection. Surgery 159:193–203
Tummers QR, Schepers A, Hamming JF, Hamming JF, Kievit J, Frangioni JV, vn de Velde CJ, Vahrmeijer AL (2015) Intraoperative guidance in parathyroid surgery using near-infrared fluorescence imaging and low-dose methylene blue. Surgery 158:1323–1330
Phitayakorn R, McHenry CR (2006) Incidence and location of ectopic abnormal parathyroid glands. Am J Surg 191:418–423
Pattou FN, Pellissier LC, Noël C, Wambergue F, Huglo DG, Proye CA (2000) Supernumerary parathyroid glands: frequency and surgical significance in treatment of renal hyperparathyroidism. World J Surg 24:1330–1334
Alander JT, Kaartinen I, Laakso A, Pätilä T, Spillmann T, Tuchin VV, Venermo M, Välisuo P (2012) A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 22:2021. doi:10.1155/2012/940585
McHenry CR, Speroff T, Wentworth D, Murphy T (1994) Risk factors for postthyroidectomy hypocalcemia. Surgery 116:641–648
Pattou F, Combemale F, Fabre S, Carnaille B, Decoulx M, Wemeau JL, Racadot A, Proye C (1998) Hypocalcemia following thyroid surgery: incidence and prediction of outcome. World J Surg 22:718–724
Sasson AR, Pingpank JF Jr, Wetherington W, Hanlon AL, Ridge JA (2001) Incidental parathyroidectomy during thyroid surgery does not cause transient symptomatic hypocalcemia. Arch Otolaryngol Head Neck Surg 127:304–308
Wingert DJ, Friesen SR, Iliopoulos JI, Pierce GE, Thomas JH, Hermreck AS (1986) Post-thyroidectomy hypocalcemia. Incidence and risk factors. Am J Surg 152:606–610
Demeester-Mirkine N, Hooghe L, Van Geertruyden J, De Maertelaer V (1992) Hypocalcemia after thyroidectomy. Arch Surg 127:854–858
Paras C, Keller M, White L, Phay J, Mahadevan-Jasen A (2011) Near-infrared autofluorescence for the detection of parathyroid glands. J Biomed Opt 16:067012
Vidal Fortuny J, Belfontali V, Sadowski SM, Karenovics W, Guigard S, Triponez F (2016) Parathyroid gland angiography with indocyanine green fluorescence to predict parathyroid function after thyroid surgery. Br J Surg 103:537–543
Chakedis JM, Maser C, Brumund KT, Bouvet M (2015) Indocyanine green fluorescence-guided redo parathyroidectomy. BMJ Case Rep. doi:10.1136/bcr-2015-211778
Zaidi N, Bucak E, Yazici P, Soundararajan S, Okoh A, Yigitbas H, Dural C, Berber E (2016) The feasibility of indocyanine green fluorescence imaging for identifying and assessing the perfusion of parathyroid glands during total thyroidectomy. J Surg Oncol 113:775–778
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Jorge Falco, Fernando Dip, Pablo Quadri, Martin de la Fuente, Marcos Prunello, and Raul J. Rosenthal have no conflicts of interest or financial ties to disclose. The equipment was provided by Fluoptics® France.
Rights and permissions
About this article
Cite this article
Falco, J., Dip, F., Quadri, P. et al. Increased identification of parathyroid glands using near infrared light during thyroid and parathyroid surgery. Surg Endosc 31, 3737–3742 (2017). https://doi.org/10.1007/s00464-017-5424-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5424-1