Abstract
Background
Detection of an incipient peritoneal carcinomatosis (PC) is still challenging, and there is a crucial need for technological improvements in order to diagnose and to treat early this condition. Fujinon Intelligent Chromo Endoscopy (FICE) is a spectral image processing technology that enhances the contrast of the target tissue. The aim of this study is to investigate the usefulness of FICE system during peritoneal endoscopy and to establish the optimal FICE preset(s) for peritoneal exploration and PC detection.
Methods
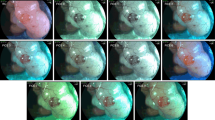
A total of 561 images corresponding to 51 different areas of PC nodules and normal peritoneum were recorded during peritoneal endoscopies (For each area, one white light endoscopy (WLE) image and 10 FICE images). Three groups of 5 evaluators each: senior surgeons, surgical residents and medical students assessed these images. In a first questionnaire, the evaluators gave a score ranging from 1 to 10 to each image, and the three best FICE channels were determined. In a second questionnaire, five criteria were studied specifically: contrast, brightness, vascular architecture, differentiation between organs and detection of PC. The evaluators ranked the WLE and the three best FICE channel images according to these criteria.
Results
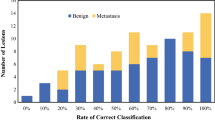
The three best FICE channels were channels 6, 2 and 9 with mean scores of 6.21 ± 1.59, 6.17 ± 1.48 and 6.06 ± 1.52, respectively. FICE Channel 2 was superior to WLE and other FICE channels, in terms of contrast (p < 10−4), visualization of vascular architecture (p < 10−4), differentiation between organs (p < 10−4) and detection of PC (p < 10−4); and ranked first in 38.8, 41.5, 31 and 46.9 % of the cases, respectively.
Conclusion
FICE system provides adequate illumination of the abdominal cavity and a unique contrast that enhances the vascular architecture. FICE Channel 2 is the optimal channel for peritoneal exploration and could be a useful tool for the diagnosis of PC during peritoneal explorations.


Similar content being viewed by others
References
Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FAN (2003) Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol Off J Am Soc Clin Oncol 21:3737–3743. doi:10.1200/JCO.2003.04.187
Elias D, Gilly F, Quenet F, Bereder JM, Sidéris L, Mansvelt B, Lorimier G, Glehen O, Association Française de Chirurgie (2010) Pseudomyxoma peritonei: a French multicentric study of 301 patients treated with cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 36:456–462. doi:10.1016/j.ejso.2010.01.006
Elias D, Lefevre JH, Chevalier J, Brouquet A, Marchal F, Classe J-M, Ferron G, Guilloit J-M, Meeus P, Goéré D, Bonastre J (2009) Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol Off J Am Soc Clin Oncol 27:681–685. doi:10.1200/JCO.2008.19.7160
Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H (2008) 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 15:2426–2432. doi:10.1245/s10434-008-9966-2
Elias D, Gilly F, Boutitie F, Quenet F, Bereder J-M, Mansvelt B, Lorimier G, Dubè P, Glehen O (2010) Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol Off J Am Soc Clin Oncol 28:63–68. doi:10.1200/JCO.2009.23.9285
Dromain C, Leboulleux S, Auperin A, Goere D, Malka D, Lumbroso J, Schumberger M, Sigal R, Elias D (2008) Staging of peritoneal carcinomatosis: enhanced CT vs. PET/CT. Abdom Imaging 33:87–93. doi:10.1007/s00261-007-9211-7
Smyth EC, Shah MA (2011) Role of 18F 2-fluoro-2-deoxyglucose positron emission tomography in upper gastrointestinal malignancies. World J Gastroenterol 17:5059–5074. doi:10.3748/wjg.v17.i46.5059
Yan TD, Morris DL, Shigeki K, Dario B, Marcello D (2008) Preoperative investigations in the management of peritoneal surface malignancy with cytoreductive surgery and perioperative intraperitoneal chemotherapy: expert consensus statement. J Surg Oncol 98:224–227. doi:10.1002/jso.21069
Angelelli G, Ianora AA, Scardapane A, Pedote P, Memeo M, Rotondo A (2001) Role of computerized tomography in the staging of gastrointestinal neoplasms. Semin Surg Oncol 20:109–121
de Bree E, Koops W, Kröger R, van Ruth S, Verwaal VJ, Zoetmulder FN (2006) Preoperative computed tomography and selection of patients with colorectal peritoneal carcinomatosis for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 32:65–71. doi:10.1016/j.ejso.2005.09.016
Elias D, Goéré D, Di Pietrantonio D, Boige V, Malka D, Kohneh-Shahri N, Dromain C, Ducreux M (2008) Results of systematic second-look surgery in patients at high risk of developing colorectal peritoneal carcinomatosis. Ann Surg 247:445–450. doi:10.1097/SLA.0b013e31815f0113
Technology Committee ASGE, Manfredi MA, Abu Dayyeh BK, Bhat YM, Chauhan SS, Gottlieb KT, Hwang JH, Komanduri S, Konda V, Lo SK, Maple JT, Murad FM, Siddiqui UD, Wallace MB, Banerjee S (2015) Electronic chromoendoscopy. Gastrointest Endosc 81:249–261. doi:10.1016/j.gie.2014.06.020
Subramanian V, Ragunath K (2014) Advanced endoscopic imaging: a review of commercially available technologies. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 12(368–376):e1. doi:10.1016/j.cgh.2013.06.015
Miyake YK, Kouzu T, Takeuchi S Development of new electronic endoscopes using the spectral images of an internal organ. In: Proceedings of the ISTSID’s thirteen color imaging conf. November 7–11, 2005. Scottsdale (Ariz), pp 261–269
Nunez MF, Sardi A, Jimenez W, Nieroda C, Sittig M, MacDonald R, Aydin N, Milovanov V, Gushchin V (2015) Port-site metastases is an independent prognostic factor in patients with peritoneal carcinomatosis. Ann Surg Oncol 22:1267–1273. doi:10.1245/s10434-014-4136-1
Pocard M (2015) Exploratory laparoscopy for carcinomatosis: discard that quiver full of trocars and use just one! J Visc Surg 152:147–148. doi:10.1016/j.jviscsurg.2015.04.004
Najah H, Lo Dico R, Grienay M, Dohan A, Dray X, Pocard M (2015) Single-incision flexible endoscopy (SIFE) for detection and staging of peritoneal carcinomatosis. Surg Endosc. doi:10.1007/s00464-015-4682-z
Meyers MA (1973) Distribution of intra-abdominal malignant seeding: dependency on dynamics of flow of ascitic fluid. Am J Roentgenol Radium Ther Nucl Med 119:198–206
Gallagher AG, Ritter EM, Lederman AB, McClusky DA, Smith CD (2005) Video-assisted surgery represents more than a loss of three-dimensional vision. Am J Surg 189:76–80. doi:10.1016/j.amjsurg.2004.04.008
Hanna G, Cuschieri A (2001) Image display technology and image processing. World J Surg 25:1419–1427
Muto M, Nakane M, Katada C, Sano Y, Ohtsu A, Esumi H, Ebihara S, Yoshida S (2004) Squamous cell carcinoma in situ at oropharyngeal and hypopharyngeal mucosal sites. Cancer 101:1375–1381. doi:10.1002/cncr.20482
Watanabe A, Taniguchi M, Tsujie H, Hosokawa M, Fujita M, Sasaki S (2009) The value of narrow band imaging for early detection of laryngeal cancer. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol Head Neck Surg 266:1017–1023. doi:10.1007/s00405-008-0835-1
Watanabe A, Taniguchi M, Tsujie H, Hosokawa M, Fujita M, Sasaki S (2009) The value of narrow band imaging for early detection of laryngeal cancer. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg 266:1017–1023. doi:10.1007/s00405-008-0835-1
Kumagai Y, Inoue H, Nagai K, Kawano T, Iwai T (2002) Magnifying endoscopy, stereoscopic microscopy, and the microvascular architecture of superficial esophageal carcinoma. Endoscopy 34:369–375. doi:10.1055/s-2002-25285
Yoshida T, Inoue H, Usui S, Satodate H, Fukami N, Kudo S (2004) Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest Endosc 59:288–295
Hu Y-Y, Lian Q-W, Lin Z-H, Zhong J, Xue M, Wang L-J (2015) Diagnostic performance of magnifying narrow-band imaging for early gastric cancer: a meta-analysis. World J Gastroenterol 21:7884–7894. doi:10.3748/wjg.v21.i25.7884
Yu H, Yang A-M, Lu X-H, Zhou W-X, Yao F, Fei G-J, Guo T, Yao L-Q, He L-P, Wang B-M (2015) Magnifying narrow-band imaging endoscopy is superior in diagnosis of early gastric cancer. World J Gastroenterol 21:9156–9162. doi:10.3748/wjg.v21.i30.9156
Chiu H-M, Chang C-Y, Chen C-C, Lee Y-C, Wu M-S, Lin J-T, Shun C-T, Wang H-P (2007) A prospective comparative study of narrow-band imaging, chromoendoscopy, and conventional colonoscopy in the diagnosis of colorectal neoplasia. Gut 56:373–379. doi:10.1136/gut.2006.099614
Su M-Y, Hsu C-M, Ho Y-P, Chen P-C, Lin C-J, Chiu C-T (2006) Comparative study of conventional colonoscopy, chromoendoscopy, and narrow-band imaging systems in differential diagnosis of neoplastic and nonneoplastic colonic polyps. Am J Gastroenterol 101:2711–2716. doi:10.1111/j.1572-0241.2006.00932.x
Schnelldorfer T, Jenkins RL, Birkett DH, Wright VJ, Price LL, Georgakoudi I (2015) Laparoscopic narrow band imaging for detection of occult cancer metastases: a randomized feasibility trial. Surg Endosc. doi:10.1007/s00464-015-4401-9
Fanfani F, Gallotta V, Rossitto C, Fagotti A, Scambia G (2010) Narrow band imaging in borderline ovarian tumor. J Minim Invasive Gynecol 17:146–147. doi:10.1016/j.jmig.2009.04.001
Fanfani F, Rossitto C, Fagotti A, Gallotta V, Gagliardi ML, Scambia G (2011) Narrow-band imaging in laparoscopic management of cervical carcinoma. J Minim Invasive Gynecol 18:146–147. doi:10.1016/j.jmig.2010.02.001
Schnelldorfer T (2012) Image-enhanced laparoscopy: a promising technology for detection of peritoneal micrometastases. Surgery 151:345–350. doi:10.1016/j.surg.2011.12.012
Ishida A, Ishikawa F, Nakamura M, Miyazu YM, Mineshita M, Kurimoto N, Koike J, Nishisaka T, Miyazawa T, Astoul P (2009) Narrow band imaging applied to pleuroscopy for the assessment of vascular patterns of the pleura. Respir Int Rev Thorac Dis 78:432–439. doi:10.1159/000247335
Schönfeld N, Schwarz C, Kollmeier J, Blum T, Bauer TT, Ott S (2009) Narrow band imaging (NBI) during medical thoracoscopy: first impressions. J Occup Med Toxicol Lond Engl 4:24. doi:10.1186/1745-6673-4-24
Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, Yoshida S, Hamamoto Y, Endo T (2004) Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt 9:568–577. doi:10.1117/1.1695563
Chaiteerakij R, Rerknimitr R, Kullavanijaya P (2010) Role of digital chromoendoscopy in detecting minimal change esophageal reflux disease. World J Gastrointest Endosc 2:121–129. doi:10.4253/wjge.v2.i4.121
Miyasaka M, Hirakawa M, Nakamura K, Tanaka F, Mimori K, Mori M, Honda H (2011) The endoscopic diagnosis of nonerosive reflux disease using flexible spectral imaging color enhancement image: a feasibility trial. Dis Esophagus Off J Int Soc Dis Esophagus ISDE 24:395–400. doi:10.1111/j.1442-2050.2010.01166.x
Camus M, Coriat R, Leblanc S, Brezault C, Terris B, Pommaret E, Gaudric M, Chryssostalis A, Prat F, Chaussade S (2012) Helpfulness of the combination of acetic acid and FICE in the detection of Barrett’s epithelium and Barrett’s associated neoplasias. World J Gastroenterol 18:1921–1925. doi:10.3748/wjg.v18.i16.1921
Qumseya BJ, Wang H, Badie N, Uzomba RN, Parasa S, White DL, Wolfsen H, Sharma P, Wallace MB (2013) Advanced imaging technologies increase detection of dysplasia and neoplasia in patients with Barrett’s esophagus: a meta-analysis and systematic review. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 11(1562–1570):e1–e2. doi:10.1016/j.cgh.2013.06.017
Arantes V, Albuquerque W, Salles JMP, Freitas Dias CA, Alberti LR, Kahaleh M, Ferrari TCA, Coelho LGV (2013) Effectiveness of unsedated transnasal endoscopy with white-light, flexible spectral imaging color enhancement, and lugol staining for esophageal cancer screening in high-risk patients. J Clin Gastroenterol 47:314–321. doi:10.1097/MCG.0b013e3182617fc1
Mouri R, Yoshida S, Tanaka S, Oka S, Yoshihara M, Chayama K (2009) Evaluation and validation of computed virtual chromoendoscopy in early gastric cancer. Gastrointest Endosc 69:1052–1058. doi:10.1016/j.gie.2008.08.032
Nakamura M, Nishikawa J, Goto A, Nishimura J, Hashimoto S, Okamoto T, Sakaida I (2013) Usefulness of ultraslim endoscopy with flexible spectral imaging color enhancement for detection of gastric neoplasm: a preliminary study. J Gastrointest Cancer 44:325–328. doi:10.1007/s12029-013-9500-z
Osawa H, Yamamoto H, Miura Y, Ajibe H, Shinhata H, Yoshizawa M, Sunada K, Toma S, Satoh K, Sugano K (2012) Diagnosis of depressed-type early gastric cancer using small-caliber endoscopy with flexible spectral imaging color enhancement. Dig Endosc Off J Jpn Gastroenterol Endosc Soc 24:231–236. doi:10.1111/j.1443-1661.2011.01224.x
Togashi K, Osawa H, Koinuma K, Hayashi Y, Miyata T, Sunada K, Nokubi M, Horie H, Yamamoto H (2009) A comparison of conventional endoscopy, chromoendoscopy, and the optimal-band imaging system for the differentiation of neoplastic and non-neoplastic colonic polyps. Gastrointest Endosc 69:734–741. doi:10.1016/j.gie.2008.10.063
Coriat R, Chryssostalis A, Zeitoun JD, Deyra J, Gaudric M, Prat F, Chaussade S (2008) Computed virtual chromoendoscopy system (FICE): a new tool for upper endoscopy? Gastroentérologie Clin Biol 32:363–369. doi:10.1016/j.gcb.2007.11.013
Osawa H, Yoshizawa M, Yamamoto H, Kita H, Satoh K, Ohnishi H, Nakano H, Wada M, Arashiro M, Tsukui M, Ido K, Sugano K (2008) Optimal band imaging system can facilitate detection of changes in depressed-type early gastric cancer. Gastrointest Endosc 67:226–234. doi:10.1016/j.gie.2007.06.067
Acknowledgments
The authors would like to thank Drs. Karine Pautrat, Romain Amato, Silvia Basato, Giulia Boarini, and our surgical residents Iris Bitumba, Louise Montalva, Florie Pirot, Pauline Dewaele, Firas Dridi and Nicolas Leguimazon for their helpful contribution to this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Fujifilm Medical System Company paid the inscription and the travel to the «United European Gastroenterology Week» which took place in Stockholm in October 2011. One member of the team was present for the poster presentation (Dr. R.L.D.). It is also a partner to the INSERM U965 Unit to study impact of endoscopy on evaluation of peritoneal carcinomatosis. Drs. L. Marry, A. Dohan, H. Najah, C. Eveno, M. Pocard have no conflicts of interest or financial ties to disclose.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00464-016-5101-9.
Rights and permissions
About this article
Cite this article
Najah, H., Lo Dico, R., Dohan, A. et al. A feasibility study of the use of computed virtual chromoendoscopy for laparoscopic evaluation of peritoneal metastases. Surg Endosc 31, 743–751 (2017). https://doi.org/10.1007/s00464-016-5028-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-5028-1