Abstract
Introduction
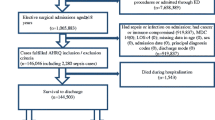
Postoperative sepsis is a rare but serious complication following elective surgery. The purpose of this study was to identify the rate of postoperative sepsis following elective laparoscopic gastric bypass (LGBP) and to identify patients’ modifiable, preoperative risk factors.
Methods
The American College of Surgeons National Surgical Quality Improvement Program database was queried from 2005 to 2013 for factors associated with the development of postoperative sepsis following elective LGBP. Patients who developed sepsis were compared to those who did not. Results were analyzed using the Chi-square test for categorical variables and Wilcoxon two-sample test for continuous variables. A multivariate logistic regression analysis was utilized to calculate adjusted odds ratios for factors contributing to sepsis.
Results
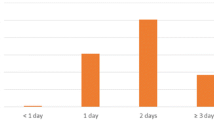
During the study period, 66,838 patients underwent LGBP. Of those, 546 patients developed postoperative sepsis (0.82 %). The development of sepsis was associated with increased operative time (161 ± 77.8 vs. 135.10 ± 56.5 min; p < 0.0001) and a greater number of preoperative comorbidities, including diabetes (39.6 vs. 30.6 %; p < 0.0001), hypertension requiring medication (65.2 vs. 54 %; p < 0.0001), current tobacco use (16.7 vs. 11.5 %; p = 0.0002), and increased pack-year history of smoking (8.6 ± 18.3 vs. 5.6 ± 14.2; p = 0.0006), and the Charlson Comorbidity Index (0.51 ± 0.74 vs. 0.35 ± 0.57, p < 0.0001). Sepsis resulted in an increased length of stay (10.1 ± 14.4 vs. 2.4 ± 4.8 days; p < 0.0001) and a 30 times greater chance of 30-day mortality (4.03 vs. 0.11 %, p < 0.0001). Multivariate logistic regression analysis showed that current smokers had a 63 % greater chance of developing sepsis compared to non-smokers, controlling for age, race, gender, BMI, and CCI score (OR 1.63, 95 % CI 1.23–2.14; p = 0.0006).
Conclusions
Laparoscopic gastric bypass is uncommonly associated with postoperative sepsis. When it occurs, it portends a 30 times increased risk of death. A patient history of diabetes, hypertension, and increasing pack-years of smoking portend an increased risk of sepsis. Current smoking status, a preoperative modifiable risk factor, is independently associated with the chance of postoperative sepsis. Preoperative patient optimization and risk reduction should be a priority for elective surgery, and patients should be encouraged to stop smoking prior to gastric bypass.
Similar content being viewed by others
References
Spaniolas K, Trus TL, Adrales GL, Quigley MT, Pories WJ, Laycock WS (2014) Early morbidity and mortality of laparoscopic sleeve gastrectomy and gastric bypass in the elderly: a NSQIP analysis. Surg Obes Relat Dis Off J Am Soc Bariatr Surg 10(4):584–588 (Epub 2014/06/11)
Khan MA, Grinberg R, Johnson S, Afthinos JN, Gibbs KE (2013) Perioperative risk factors for 30-day mortality after bariatric surgery: is functional status important? Surg Endosc 27(5):1772–1777 (Epub 2013/01/10)
Nandipati K, Lin E, Husain F, Perez S, Srinivasan J, Sweeney JF et al (2013) Factors predicting the increased risk for return to the operating room in bariatric patients: a NSQIP database study. Surg Endosc 27(4):1172–1177 (Epub 2012/10/19)
Gupta PK, Franck C, Miller WJ, Gupta H, Forse RA (2011) Development and validation of a bariatric surgery morbidity risk calculator using the prospective, multicenter NSQIP dataset. J Am Coll Surg 212(3):301–309 (Epub 2011/01/21)
Sanni A, Perez S, Medbery R, Urrego HD, McCready C, Toro JP et al (2014) Postoperative complications in bariatric surgery using age and BMI stratification: a study using ACS-NSQIP data. Surg Endosc 28(12):3302–3309 (Epub 2014/08/15)
Bruschi Kelles SM, Machado CJ, Barreto SM (2014) Mortality rate after open Roux-in-Y gastric bypass: a 10-year follow-up. Braz J Med Biol Res 47(7):617–625
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Birim O, Maat AP, Kappetein AP, van Meerbeeck JP, Damhuis RA, Bogers AJ (2003) Validation of the Charlson comorbidity index in patients with operated primary non-small cell lung cancer. Eur J Cardiothorac Surg 23(1):30–34
Abdollah F, Sun M, Schmitges J, Thuret R, Djahangirian O, Jeldres C et al (2012) Development and validation of a reference table for prediction of postoperative mortality rate in patients treated with radical cystectomy: a population-based study. Ann Surg Oncol 19(1):309–317
Schroeder RA, Marroquin CE, Bute BP, Khuri S, Henderson WG, Kuo PC (2006) Predictive indices of morbidity and mortality after liver resection. Ann Surg 243(3):373–379
Ross SW, Oommen B, Kim M, Walters AL, Green JM, Heniford BT et al (2014) A little slower, but just as good: postgraduate year resident versus attending outcomes in laparoscopic ventral hernia repair. Surg Endosc 28(11):3092–3100
Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA (2004) New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 57(12):1288–1294
D’Hoore W, Bouckaert A, Tilquin C (1996) Practical considerations on the use of the Charlson comorbidity index with administrative data bases. J Clin Epidemiol 49(12):1429–1433
Ehlert BA, Durham CA, Parker FM, Bogey WM, Powell CS, Stoner MC (2011) Impact of operative indication and surgical complexity on outcomes after thoracic endovascular aortic repair at National Surgical Quality Improvement Program Centers. J Vasc Surg 54(6):1629–1636
Bergman S, Martelli V, Monette M, Sourial N, Deban M, Hamadani F et al (2013) Identification of quality of care deficiencies in elderly surgical patients by measuring adherence to process-based quality indicators. J Am Coll Surg 217(5):858–866
Cawley J, Sweeney MJ, Kurian M, Beane S (2007) Predicting complications after bariatric surgery using obesity-related co-morbidities. Obes Surg 17(11):1451–1456
Ramos M, Khalpey Z, Lipsitz S, Steinberg J, Panizales MT, Zinner M et al (2008) Relationship of perioperative hyperglycemia and postoperative infections in patients who undergo general and vascular surgery. Ann Surg 248(4):585–591
Steele KE, Prokopowicz GP, Chang HY, Richards T, Clark JM, Weiner JP et al (2012) Risk of complications after bariatric surgery among individuals with and without type 2 diabetes mellitus. Surg Obes Relat Dis Off J Am Soc Bariatr Surg 8(3):305–330
Colavita PD, Zemlyak AY, Burton PV, Dacey KT, Walters AL, Lincourt AE, Tsirline VE, Kercher KW, Heniford BT (eds) (2013) The expansive cost of wound complications after ventral hernia repair. American College of Surgeons, Washington
Padwal RS, Klarenbach SW, Wang X, Sharma AM, Karmali S, Birch DW et al (2013) A simple prediction rule for all-cause mortality in a cohort eligible for bariatric surgery. JAMA Surg 148(12):1109–1115 (Epub 2013/10/18)
Marchini JF, Souza FL, Schmidt A, Cunha SF, Salgado W Jr, Marchini JS et al (2012) Low educational status, smoking, and multidisciplinary team experience predict hospital length of stay after bariatric surgery. Nutr Metab Insights 5:71–76 (Epub 2012/01/01)
Lent MR, Hayes SM, Wood GC, Napolitano MA, Argyropoulos G, Gerhard GS et al (2013) Smoking and alcohol use in gastric bypass patients. Eat Behav 14(4):460–463 (Epub 2013/11/05)
Lindstrom D, Sadr Azodi O, Wladis A, Tonnesen H, Linder S, Nasell H et al (2008) Effects of a perioperative smoking cessation intervention on postoperative complications: a randomized trial. Ann Surg 248(5):739–745 (Epub 2008/10/25)
Sørensen LT, Jørgensen T (2003) Short-term pre-operative smoking cessation intervention does not affect postoperative complications in colorectal surgery: a randomized clinical trial. Colorectal Dis 5(4):347–352
Moller AM, Villebro N, Pedersen T, Tonnesen H (2002) Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet 359(9301):114–117 (Epub 2002/01/26)
Reinbold C, Rausky J, Binder JP, Revol M (2014) Urinary cotinine testing as pre-operative assessment of patients undergoing free flap surgery. Ann Chir Plast Esthet. 2Epub pii: S0294-1260(14)00191-5. doi:10.1016/j.anplas.2014.10.002. (Epub ahead of print)
Catanzarite T, Saha S, Pilecki MA, Kim JY, Milad MP (2015) Longer operative time during benign laparoscopic and robotic hysterectomy is associated with increased 30-day perioperative complications. J Minim Invasive Gynecol. doi:10.1016/j.jmig.2015.05.022. (Epub ahead of print)
Bailey MB, Davenport DL, Vargas HD, Evers BM, McKenzie SP (2014) Longer operative time: deterioration of clinical outcomes of laparoscopic colectomy versus open colectomy. Dis Colon Rectum 57(5):616–622
Wadgaonkar AD, Schneider EC, Bhattacharyya T (2010) Physician tiering by health plans in Massachusetts. J Bone Joint Surg Am 92(12):2204–2209
Hong CS, Atlas SJ, Chang Y, Subramanian SV, Ashburner JM, Barry MJ et al (2010) Relationship between patient panel characteristics and primary care physician clinical performance rankings. JAMA 304(10):1107–1113
Sugerman HJ, Wolfe LG, Sica DA, Clore JN (2003) Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg 237(6):751–756 (discussion 7–8)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Heniford, Augenstein, and Lincourt have previously been awarded surgical research and education grants from W.L. Gore and Associates, Ethicon, Novadaq, Bard/Davol, and LifeCell Inc. Vedra A. Augenstein MD, FACS, has previously been awarded surgical research and education grants from Bard, Ethicon and Lifecell. L. J. Blair, C. R. Huntington, T. C. Cox, T. Prasad, A. E. Lincourt, and K. S. Gersin have no potential conflict or disclosures relevant to this work.
Rights and permissions
About this article
Cite this article
Blair, L.J., Huntington, C.R., Cox, T.C. et al. Risk factors for postoperative sepsis in laparoscopic gastric bypass. Surg Endosc 30, 1287–1293 (2016). https://doi.org/10.1007/s00464-015-4349-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4349-9