Abstract
Introduction
MMP-3, a member of the matrix metalloproteinase (MMP) family, is involved in the breakdown of the extracellular matrix in tissue remodeling and may also play a role in cancer progression and metastasis. Minimally invasive colorectal resection (MICR) may increase plasma MMP-3 levels directly via surgical trauma or indirectly due to surgery-associated elevations in TNF-α and IL1 which are regulators of MMP-3. This study’s purpose was to evaluate plasma MMP-3 levels during the first month after MICR for colorectal cancer.
Methods
Patients enrolled in an IRB approved data/plasma bank who underwent elective MICR for CRC. Blood plasma samples had been collected preoperatively, on postoperative day (POD) 1, 3 and at varying postoperative time points and were stored at −80 °C. The late samples (POD 7–41) were bundled into 7 day time blocks and considered as single time points. MMP-3 levels were analyzed in duplicate via ELISA and the results reported as mean ± SD. The paired t test was used for analysis (significance, p < 0.008 after Bonferroni’s correction).
Results
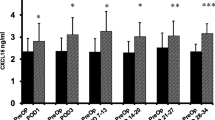
A total of 73 CRC patients who underwent MICR met the inclusion criteria. The mean PreOp MMP-3 level was 14.9 ± 7.8 ng/ml (n = 73). Significantly elevated mean plasma levels were noted on POD 1 (21.4 ± 14.7 ng/ml, n = 73, p < 0.0001), POD 3 (37.9 ± 21.5 ng/ml, n = 72, p < 0.0001), POD 7–13 (22.0 ± 13.0 ng/ml, n = 56, p < 0.0001), POD 14–20 (21.9 ± 10.3 ng/ml, n = 20, p = 0.003), and on POD 21–27 (21.9 ± 11.43 ng/ml, n = 20, p = 0.002) when compared to PreOp levels. Plasma levels returned to the PreOp baseline at the POD 28–41 time point (n = 16, p = 0.07).
Conclusion
Plasma MMP-3 levels remained significantly elevated from baseline for 4 weeks after MICR for CRC. The early postoperative increase in MMP-3 levels may be due to the surgery-related acute inflammatory response; the elevation noted during weeks 2–3 may be related to wound healing. Increased MMP-3 levels may promote metastases or the growth of residual cancer.

Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30
Gutman M, Fidler IJ (1995) Biology of human colon cancer metastasis. World J Surg 19:226–234
Allendorf JD, Bessler M, Horvath KD, Marvin MR, Laird DA, Whelan RL (1998) Increased tumor establishment and growth after open vs laparoscopic bowel resection in mice. Surg Endosc 12:1035–1038
Carter JJ, Feingold DL, Kirman I, Oh A, Wildbrett P, Asi Z, Fowler R, Huang E, Whelan RL (2003) Laparoscopic-assisted cecectomy is associated with decreased formation of postoperative pulmonary metastases compared with open cecectomy in a murine model. Surgery 134:432–436
Allendorf JD, Bessler M, Kayton ML, Oesterling SD, Treat MR, Nowygrod R, Whelan RL (1995) Increased tumor establishment and growth after laparotomy vs. laparoscopy in a murine model. Arch Surg 130:649–653
Peeters CF, de Waal RM, Wobbes T, Westphal JR, Ruers TJ (2006) Outgrowth of human liver metastases after resection of the primary colorectal tumor: a shift in the balance between apoptosis and proliferation. Int J Cancer 119:1249–1253
Lange P, Hekmat K, Bosl G, Kennedy BJ, Fraley EE (1980) Accelerated growth of testicular cancer after cytoreductive surgery. Cancer 45:1498–1506
Crawford SE, Flores-Stadler EM, Huang L, Tan XD, Ranalli M, Mu Y, Gonzalez-Crussi F (1998) Rapid growth of cutaneous metastases after surgical resection of thrombospondin-secreting small blue round cell tumor of childhood. Hum Pathol 29:1039–1044
Ben-Eliyahu S (2003) The promotion of tumor metastasis by surgery and stress: immunological basis and implications for psychoneuroimmunology. Brain Behav Immun 17(Suppl 1):S27–S36
Allendorf JD, Bessler M, Horvath KD, Marvin MR, Laird DA, Whelan RL (1999) Increased tumor establishment and growth after open versus laparoscopic surgery in mice may be related to differences in postoperative T-cell function. Surg Endosc 13:233–235
Shantha Kumara HMC, Feingold D, Kalady M, Dujovny N, Senagore A, Hyman N, Cekic V, Whelan RL (2009) Colorectal resection is associated with persistent proangiogenic plasma protein changes: postoperative plasma stimulates in vitro endothelial cell growth, migration, and invasion. Ann Surg 249:973–977 Erratum in: 250:1046
Belizon A, Balik E, Horst P, Feingold D, Arnell T, Azarani T, Cekic V, Skitt R, Kumara S, Whelan RL (2008) Persistent elevation of plasma vascular endothelial growth factor levels during the first month after minimally invasive colorectal resection. Surg Endosc 22:287–297
Shantha Kumara HMC, Cabot JC, Yan X, Herath SA, Luchtefeld M, Kalady MF, Feingold DL, Baxter R, Whelan RL (2011) Minimally invasive colon resection is associated with a persistent increase in plasma PlGF levels following cancer resection. Surg Endosc 25:2153–2158
Shantha Kumara HMC, Tohme ST, Herath SA, Yan X, Senagore AJ, Nasar A, Kalady MF, Baxter R, Whelan RL (2012) Plasma soluble vascular adhesion molecule-1 levels are persistently elevated during the first month after colorectal cancer resection. Surg Endosc 26:1759–1764
Shantha Kumara HMC, Kirchoff D, Naffouje S, Grieco M, Herath SA, Dujovny N, Kalady MF, Hyman N, Njoh L, Whelan RL (2012) Plasma from the second and third weeks after open colorectal resection for cancer stimulates in vitro endothelial cell growth, migration, and invasion. Surg Endosc 26:790–795
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285:1182–1186
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Orlichenko LS, Radisky DC (2008) Matrix metalloproteinases stimulate epithelial-mesenchymal transition during tumor development. Clin Exp Metastasis 25:593–600
Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ (1997) Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol 139:1861–1872
Mendes O, Kim HT, Stoica G (2005) Expression of MMP2, MMP9 and MMP-3 in breast cancer brain metastasis in a rat model. Clin Exp Metastasis 22:237–246
Radisky ES, Radisky DC (2010) Matrix metalloproteinase induced epithelial-mesenchymal transition in breast cancer. J Mammary Gland Biol Neoplasia 15:201–212
Lee EJ, Kim SY, Hyun JW, Min SW, Kim DH, Kim HS (2010) Glycitein inhibits glioma cell invasion through down-regulation of MMP-3 and MMP-9 gene expression. Chem Biol Interact 185:18–24
Jung K, Nowak L, Lein M, Priem F, Schnorr D, Loening SA (1997) Matrix metalloproteinases 1 and 3, tissue inhibitor of metalloproteinases1 and the complex of metalloproteinases-1/tissue inhibitor in plasma of patients with prostate cancer. Int J Cancer 74:220–223
Tang CH, Yamamoto A, Lin YT, Fong YC, Tan TW (2010) Involvement of matrix metalloproteinases-3 in CCL5/CCR5 pathway of chondrosarcoma metastasis. Biochem Pharmacol 79:209–217
Ishii Y, Nakasato Y, Kobayashi S, Yamazaki Y, Aoki T (2003) A study on angiogenesis-related matrix metalloproteinase networks in primary hepatocellular carcinoma. J Exp Clin Cancer Res 22:461–470
Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z (1999) The stromal proteinase MMP-3/Stromelysin-1 promotes mammary carcinogenesis. Cell 98:137–146
Liu HQ, Song S, Wang JH, Zhang SL (2011) Expression of MMP-3 and TIMP-3 in gastric cancer tissue and its clinical significance. Oncol Lett 2:1319–1322
Yeh YC, Sheu BS, Cheng HC, Wang YL, Yang HB, Wu JJ (2010) Elevated serum matrix metalloproteinase-3 and -7 in H. pylori-related gastric cancer can be biomarkers correlating with a poor survival. Dig Dis Sci 55:1649–1657
Okamoto K, Ishida C, Ikebuchi Y, Mandai M, Mimura K, Murawaki Y, Yuasa I (2010) The genotypes of IL-1 beta and MMP-3 are associated with the prognosis of HCV-related hepatocellular carcinoma. Intern Med 49:887–895
Wu FPK, Hoekman K, Meijer S, Cuesta MA (2003) VEGF and endostatin levels in wound fluid and plasma after breast surgery. Angiogenesis 6:255–257
Karayiannakis AJ, Zbar A, Polychronidis A, Simopoulos C (2003) Serum and drainage fluid vascular endothelial growth factor levels in early surgical wounds. Eur Surg 35:492–496
Wu FP, Hoekman K, Sietses C, von Blomberg BM, Meijer S, Bonjer HJ, Cuesta MA (2004) Systemic and peritoneal angiogenic response after laparoscopic or conventional colon resection in cancer patients: a prospective, randomized trial. Dis Colon Rectum 47:1670–1674
Katz MH, Alvarez AF, Kirsner RS, Eaglstein WH, Falanga V (1991) Human wound fluid from acute wounds stimulates fibroblast and endothelial cell growth. J Am Acad Dermatol 25:1054–1058
Bullard KM, Lund L, Mudgett JS, Mellin TN, Hunt TK, Murphy B, Ronan J, Werb Z, Banda MJ (1999) Impaired wound contraction in stromelysin-1 deficient mice. Ann Surg 230:260–265
Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML (2005) Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol 169:681–691
Sage EH, Reed M, Funk SE, Truong T, Steadele M, Puolakkainen P, Maurice DH, Bassuk JA (2003) Cleavage of the matricellular protein SPARC by matrix metalloproteinase 3 produces polypeptides that influence angiogenesis. J Biol Chem 278:37849–37857
Jin X, Yagi M, Akiyama N, Hirosaki T, Higashi S, Lin CY, Dickson RB, Kitamura H, Miyazaki K (2006) Matriptase activates stromelysin (MMP-3) and promotes tumor growth and angiogenesis. Cancer Sci 97:1327–1334
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139:871–890
Yamamura Y, Matsuzaki H, Seo K (1998) Early local recurrence of rectal cancer showing extremely rapid growth after curative surgery: report of a case. Jpn J Surg 28:1175–1178
Shantha Kumara HM, Kirman I, Feingold D, Cekic V, Nasar A, Arnell T, Balik E, Hoffman A, Baxter R, Conte S, Whelan RL (2009) Perioperative GMCSF limits the proangiogenic plasma protein changes associated with colorectal cancer resection. Eur J Surg Oncol 35:295–301
Mels AK, Statius Muller MG, van Leeuwen PA, von Blomberg BM, Scheper RJ, Cuesta MA, Beelen RH, Meijer S (2001) Immune-stimulating effects of low-dose perioperative recombinant granulocyte-macrophage colony-stimulating factor in patients operated on for primary colorectal carcinoma. Br J Surg 88:539–544
Ceelen W, Pattyn P, Mareel M (2014) Surgery, wound healing, and metastasis: recent insights and clinical implications. Crit Rev Oncol Hematol 89:16–26
Acknowledgments
This study was made possible by a generous donation from Mr. Wade Thompson and family to the Divisions of Colon and Rectal surgery, Department of Surgery, St Luke’s Roosevelt Hospital, New York.
Disclosures
All authors (HMC Shantha Kumara PhD, David J Gaita BS, Hiromichi Miyagaki MD, PhD, Xiaohong Yan PhD, Sonali AC Herath BS, Vesna Cekic RN, and Richard L Whelan MD) have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shantha Kumara, H.M.C., Gaita, D.J., Miyagaki, H. et al. Minimally invasive colorectal resection is associated with significantly elevated levels of plasma matrix metalloproteinase 3 (MMP-3) during the first month after surgery which may promote the growth of residual metastases. Surg Endosc 28, 3322–3328 (2014). https://doi.org/10.1007/s00464-014-3612-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-014-3612-9