Abstract
Background
One of the main concerns of natural orifice surgery is the local and systemic impact on physiology. Few studies have compared natural orifice transluminal endoscopic surgery (NOTES) with other surgical modalities. Most studies are based on systemic variables such as postoperative serum cytokines, with conflicting results. Surgical trauma induces an early inflammatory response, release of cytokines, and local leukocyte activation and oxidative burst. Major surgical trauma is related to impairment of phagocytic function and an increase in production of active oxygen species by phagocytes. The aim of this study was to evaluate the impact of transgastric peritoneoscopy on peritoneal innate immune response compared with laparoscopy and laparotomy in swine.
Methods
Thirty-four male Sus scrofa domesticus swine were assigned to four groups: transgastric peritoneoscopy (13), laparoscopy (7), laparotomy (7), and sham procedure (7). Twenty-four hours after the procedure, peritoneal fluid cells were harvested by peritoneal washing after necropsy. Flow cytometry analysis of labeled S. aureus and E. coli phagocytosis by peritoneal neutrophils and macrophages was blindly performed. Oxidative burst activity measured by H2O2 production under different challenges was also evaluated.
Results
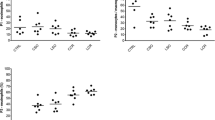
Total operative time varied between all groups. The transgastric, laparoscopy, and laparotomy groups required 56, 17.2, and 40.3 min of mean operative time, respectively (p < 0.05). Even though the mean percentage and intensity of phagocytosis by peritoneal phagocytes were higher in the sham, transgastric, and laparoscopy groups, there was no significant difference between these groups and laparotomy. Macrophage production of H2O2 has been shown to be similar among the transgastric, laparoscopy, and sham groups, and smaller than that in laparotomy (p < 0.05), either under basal conditions, while performing E. coli phagocytosis, or challenged by the presence of E. coli membrane lipopolysaccharide.
Conclusion
Under the conditions of this study, transgastric peritoneoscopy has been shown to have minimal impact on peritoneal innate immune response.




Similar content being viewed by others
References
Rattner D (2006) Introduction to NOTES White Paper. Surg Endosc 20:185
ASGE; SAGES (2006) ASGE/SAGES Working Group on natural orifice translumenal endoscopic surgery White Paper October 2005. Gastrointest Endosc 63:199–203
Bingener J, Krishnegowda NK, Michalek JE (2009) Immunologic parameters during NOTES compared with laparoscopy in a randomized blinded porcine trial. Surg Endosc 23:178–181
Dubcenco E, Assumpcao L, Dray X, Gabrielson KL, Ruben DS, Pipitone LJ, Donatelli G, Krishnamurty DM, Baker JP, Marohn MR, Kalloo AN (2009) Adhesion formation after peritoneoscopy with liver biopsy in a survival porcine model: comparison of laparotomy, laparoscopy, and transgastric natural orifice transluminal endoscopic surgery (NOTES). Endoscopy 41:971–978
McGee MF, Schomisch SJ, Marks JM, Delaney CP, Jin J, Williams C, Chak A, Matteson DT, Andrews J, Ponsky JL (2008) Late phase TNF-alpha depression in natural orifice translumenal endoscopic surgery (NOTES) peritoneoscopy. Surgery 143:318–328
Trunzo JA, McGee MF, Cavazzola LT, Schomisch S, Nikfarjam M, Bailey J, Mishra T, Poulose BK, Lee Y-J, Ponsky JL, Marks JM (2010) Peritoneal inflammatory response of natural orifice translumenal endoscopic surgery (NOTES) versus laparoscopy with carbon dioxide and air pneumoperitoneum. Surgical Endosc 24:1727–1736
Shijo H, Iwabuchi K, Hosoda S, Watanabe H, Nagaoka I, Sakakibara N (1998) Evaluation of neutrophil functions after experimental abdominal surgical trauma. Inflamm Res 47:67–74
Vittimberga F, Foley D, Meyers W, Callery M (1998) Laparoscopic surgery and the systemic immune response. Ann Surg 227:326–334
Yoshizumi F, Yasuda K, Kawaguchi K, Suzuki K, Shiraishi N, Kitano S (2009) Submucosal tunneling using endoscopic submucosal dissection for peritoneal access and closure in natural orifice transluminal endoscopic surgery: a porcine survival study. Endoscopy 41:707–711
Busque P, Higgins R, Sénéchal S, Marchand R, Quessy S (1998) Simultaneous flow cytometric measurement of Streptococcus suis phagocytosis by polymorphonuclear and mononuclear blood leukocytes. Vet Microbiol 63:229–238
Hasui M, Hirabayashi Y, Kobayashi Y (1989) Simultaneous measurement by flow cytometry of phagocytosis and hydrogen peroxide production of neutrophils in whole blood. J Immunol Methods 117:53–58
Underhill D, Ozinsky A (2002) Phagocytosis of microbes: complexity in action. Annu Rev Immunol 20:825–852
Babior B (1984) The respiratory burst of phagocytes. J Clin Invest 73:599–601
Rattner D, Hawes R (2006) NOTES: gathering momentum. Gastrointest Endosc 63:838–839
Fish E (2008) The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol 8(9):737–744
Choudhry M, Bland K, Chaudry I (2007) Trauma and immune response–effect of gender differences. Injury 38:1382–1391
Soeters P, Grimble R (2009) Dangers and benefits of the cytokine mediated response to injury and infection. Clin Nutr 28:583–596
Hazey J, Narula V, Renton D, Reavis K, Paul C, Hinshaw K, Muscarella P, Ellison E, Melvin W (2008) Natural-orifice transgastric endoscopic peritoneoscopy in humans: Initial clinical trial. Surg Endosc 22(1):16–20
Holzer K, Konietzny P, Wilhelm K, Encke A, Henrich D (2002) Phagocytosis by emigrated, intra-abdominal neutrophils is depressed during human secondary peritonitis. Eur Surg Res 34:275–284
Redmond H, Hofmann K, Shou J, Leon P, Kelly C, Daly J (1992) Effects of laparotomy on systemic macrophage function. Surgery 111:647–655
Smith J (1994) Neutrophils, host defense, and inflammation: a double-edged sword. J Leuk Biol 56:672–686
Lehrer R, Ganz T, Selsted M, Babior B, Curnutte J (1988) Neutrophils and host defense. Ann Intern Med 109:127–142
Horton J, Walker P (1993) Oxygen radicals, lipid peroxidation, and permeability changes after intestinal ischemia and reperfusion. J Appl Physiol 74:1515–1520
Bentes de Souza A, Rogers M, Wang C, Yuen P, Ng P (2003) Comparison of peritoneal oxidative stress during laparoscopy and laparotomy. J Am Assoc Gynecol Laparosc 10:65–74
Menger M, Vollmar B (2004) Surgical trauma: hyperinflammation versus immunosuppression? Langenbecks Arch Surg 389:475–484
Disclosures
Drs. R. Rodrigues, M. Rezende, G. Gomes, F. Souza, M. Blagitz, A. Della Libera, M. Taha, A. Ferrari, and E. Della Libera have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodrigues, R., Rezende, M., Gomes, G. et al. Effect of transgastric peritoneal access on peritoneal innate cellular immunity: experimental study in swine. Surg Endosc 27, 964–970 (2013). https://doi.org/10.1007/s00464-012-2541-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-012-2541-8