Abstract
Background
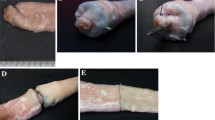
With advances in endoscopic diagnosis of early-stage gastrointestinal pathology, the need to offer minimally invasive treatment is attracting significant interest. It is essential that secure and consistent closure of full-thickness colonic resections and colotomy access be provided in natural orifice translumenal surgery (NOTES). This ex vivo porcine study aimed to evaluate a novel prototype flexible endoscopic stapler device for use in closure of full-thickness colonic defects.
Methods
A feasibility study using ex vivo porcine colon was undertaken to explore the potential of the prototype stapler to close and seal a colotomy. A standardized linear colotomy was created in 30 colons. The novel flexible endostapler was used to close 20, interrupted hand-sewn sutures to close 5, and a well-validated linear stapler to close 5 of these colotomies. The colons were subsequently subjected to leak pressure testing.
Results
The colotomy closure using the prototype stapler endoluminally required a median time of 280 s. No statistically significant difference in leak pressures between the stapler and the other techniques was described. Although the endostapler without any colotomy was found to have the highest median leak pressures and the interrupted sutures the lowest pressures, no significant difference could be demonstrated (p = 0.52). Furthermore, no significant difference was demonstrated when the closure integrity created by the flexible stapler was compared with that created by the well-validated linear stapler.
Conclusion
The results suggest that the flexible endoscopic stapler is an effective device for the safe closure of a visceral defect, which in this feasibility study was equivalent to other well-established techniques. Further studies will focus on in vivo application of the prototype stapling device in the setting of full-thickness colonic resection.







Similar content being viewed by others
References
Marescaux J, Dallemagne B, Perretta S, Wattiez A, Mutter D, Coumaros D (2007) Surgery without scars: report of transluminal cholecystectomy in a human being. Arch Surg 142:823–826 (discussion 826-827)
Iizuka H, Okamura S, Onozato Y, Ishihara H, Kakizaki S, Mori M (2009) Endoscopic submucosal dissection for colorectal tumors. Gastroenterol Clin Biol 33:1004–1011
Puli SR, Kakugawa Y, Gotoda T, Antillon D, Saito Y, Antillon MR (2009) Meta-analysis and systematic review of colorectal endoscopic mucosal resection. World J Gastroenterol 15:4273–4277
Clark J, Ziprin P (2008) Local excision and transanal endoscopic microsurgery in the management of rectal cancer with a focus on early carcinoma. Future Oncol 4:113–124
Lee W, Lee D, Choi S, Chun H (2003) Transanal endoscopic microsurgery and radical surgery for T1 and T2 rectal cancer. Surg Endosc 17:1283–1287
Lezoche G, Baldarelli M, Guerrieri M, Paganini AM, De Sanctis A, Bartolacci S, Lezoche E (2008) A prospective randomized study with a 5-year minimum follow-up evaluation of transanal endoscopic microsurgery versus laparoscopic total mesorectal excision after neoadjuvant therapy. Surg Endosc 22(2):352–358
ASGE/SAGES (2006) ASGE/SAGES Working Group on Natural Orifice Translumenal Endoscopic Surgery White Paper October 2005. Gastrointest Endosc 63:199–203
Sodergren MH, Clark J, Athanasiou T, Teare J, Yang GZ, Darzi A (2009) Natural orifice translumenal endoscopic surgery: critical appraisal of applications in clinical practice. Surg Endosc 23(4):680–687
Clark J, Sodergren M, Noonan D, Darzi A, Yang GZ (2009) The natural orifice simulated surgical environment (NOSsE): exploring the challenges of NOTES without the animal model. J Laparoendosc Adv Surg Tech A 19:211–214
Ryou M, Fong DG, Pai RD, Rattner DW, Thompson CC (2008) Transluminal closure for NOTES: an ex vivo study comparing leak pressures of various gastrotomy and colotomy closure modalities. Endoscopy 40:432–436
Sporn E, Bachman SL, Miedema BW, Loy TS, Calaluce R, Thaler K (2008) Endoscopic colotomy closure for natural orifice transluminal endoscopic surgery using a T-fastener prototype in comparison to conventional laparoscopic suture closure. Gastrointest Endosc 68:724–730
Dray X, Gabrielson KL, Buscaglia JM, Shin EJ, Giday SA, Surti VC, Assumpcao L, Marohn MR, Magno P, Pipitone LJ, Redding SK, Kalloo AN, Kantsevoy SV (2008) Air and fluid leak tests after NOTES procedures: a pilot study in a live porcine model (with videos). Gastrointest Endosc 68:513–519
Hookey LC, Khokhotva V, Bielawska B, Samis A, Jalink D, Hurlbut D, Mercer D (2009) The Queen’s closure: a novel technique for closure of endoscopic gastrotomy for natural orifice transluminal endoscopic surgery. Endoscopy 41:149–153
Voermans RP, Worm AM, van Berge Henegouwen MI, Breedveld P, Bemelman WA, Fockens P (2008) In vitro comparison and evaluation of seven gastric closure modalities for natural orifice transluminal endoscopic surgery (NOTES). Endoscopy 40:595–601
McGee MF, Marks JM, Jin J, Williams C, Chak A, Schomisch SJ, Andrews J, Okada S, Ponsky JL (2008) Complete endoscopic closure of gastric defects using a full-thickness tissue plicating device. J Gastrointest Surg 12:38–45
Meireles OR, Kantsevoy SV, Assumpcao LR, Magno P, Dray X, Giday SA, Kalloo AN, Hanly EJ, Marohn MR (2008) Reliable gastric closure after natural orifice translumenal endoscopic surgery (NOTES) using a novel automated flexible stapling device. Surg Endosc 22:1609–1613
Magno P, Giday SA, Dray X, Chung SS, Cotton PB, Gostout CJ, Hawes RH, Kalloo AN, Pasricha PJ, White JJ, Assumpcao L, Marohn MR, Gabrielson KL, Kantsevoy SV (2007) A new stapler-based full-thickness transgastric access closure: results from an animal pilot trial. Endoscopy 39:876–880
Ryou M, Pai RD, Sauer JS, Rattner DW, Thompson CC (2007) Evaluating an optimal gastric closure method for transgastric surgery. Surg Endosc 21:677–680
Disclosures
John Beardsley, Ted Bryant, and Kenneth Horton are employees of Covidien and were involved in the development of this prototype device. No specific financial support nor financial incentive was allocated to this study or persons involved by Covidien. Mikael Sodergren, James Clark, Ara Darzi, and Julian Teare have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sodergren, M., Clark, J., Beardsley, J. et al. A novel flexible endoluminal stapling device for use in NOTES colotomy closure: a feasibility study using an ex vivo porcine model. Surg Endosc 25, 3266–3272 (2011). https://doi.org/10.1007/s00464-011-1703-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-011-1703-4