Abstract
Background
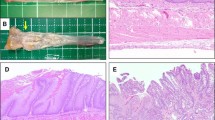
A rodent model of gastroduodenal–esophageal reflux can result in replacement of squamous esophageal mucosa with intestinal-type columnar mucosa and carcinoma. The validity of this model is debated, as it is unproven whether this mucosa is intestinal metaplasia due to reflux or represents migration of adjacent jejunal mucosa above the anastomosis. The aim of this study was to evaluate the esophageal intestinal-type mucosa in these animals by measuring expression of trefoil factor genes (TFF-1, -2, -3) and comparing it with adjacent jejunum in order to determine its etiology.
Methods
Twenty-five rats underwent esophagojejunostomy at the ligament of Treitz to induce reflux of gastric and duodenal contents. The animals were sacrificed at 16 weeks (n = 14) and 30 weeks (n = 11). After sacrifice, the distal esophagus, jejunum, and colon were obtained. RNA was isolated, reverse transcribed, and messenger RNA (mRNA) expression of TFF-1, -2, and -3 was measured with real-time polymerase chain reaction (PCR). Linear discriminant analysis classified samples based on gene expression.
Results
Esophageal intestinal-type mucosa was present at sacrifice in 18 animals. Compared to jejunum, the expression of TFF-1 and TFF-2 mRNA in the intestinal mucosa of the distal esophagus was increased (p = 0.0007 and p < 0.0001, respectively). Expression of TFF-3 was also increased in esophageal intestinal mucosa compared with jejunum (p = 0.0002), but there was significant overlap in expression between these tissues for this gene. Linear discriminant analysis misclassified esophageal intestinal-type mucosa as jejunum in only one case. In no cases was jejunum misclassified as esophageal intestinal-type mucosa.
Conclusion
The gene expression profile of esophageal intestinal-type mucosa following surgically induced reflux in a rodent model indicates that this represents intestinal metaplasia, not proximal migration of jejunum. This validates this model for studying the pathogenesis of Barrett’s esophagus. Use of this model has potential for assessment of the impact of various therapies on the natural history of reflux disease.







Similar content being viewed by others
References
Pohl H, Welch HG (2005) The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst 97(2):142–146
Kubo A, Corley DA (2004) Marked multi-ethnic variation of esophageal and gastric cardia carcinomas within the United States. Am J Gastroenterol 99(4):582–588
van Blankenstein M, Looman CWN, Johnston BJ, Caygill CPJ (2005) Age and sex distribution of the prevalence of Barrett’s esophagus found in a primary referral endoscopy center. Am J Gastroenterol 100(3):568–576
van Soest EM, Dieleman JP, Siersema PD et al (2005) Increasing incidence of Barrett’s oesophagus in the general population. Gut 54(8):1062–1066
Oberg S, Johansson J, Wenner J, Walther B (2002) Metaplastic columnar mucosa in the cervical esophagus after esophagectomy. Ann Surg 235(3):338–345
Theisen J, Nigro JJ, DeMeester TR et al (2004) Chronology of the Barrett’s metaplasia-dysplasia-carcinoma sequence. Dis Esophag 17(1):67–70
Miwa K, Sahara H, Segawa M et al (1996) Reflux of duodenal or gastro-duodenal contents induces esophageal carcinoma in rats. Int J Cancer 67(2):269–274
Attwood SE, Smyrk TC, DeMeester TR et al (1992) Duodenoesophageal reflux and the development of esophageal adenocarcinoma in rats. Surgery 111(5):503–510
Ireland AP, Peters JH, Smyrk TC et al (1996) Gastric juice protects against the development of esophageal adenocarcinoma in the rat. Ann Surg 224(3):358–370, discussion 370–371
Fein M, Peters JH, Chandrasoma P et al (1998) Duodenoesophageal reflux induces esophageal adenocarcinoma without exogenous carcinogen. J Gastrointest Surg 2(3):260–268
Nishijima K, Miwa K, Miyashita T et al (2004) Impact of the biliary diversion procedure on carcinogenesis in Barrett’s esophagus surgically induced by duodenoesophageal reflux in rats. Ann Surg 240(1):57–67
Fein M, Ireland AP, Ritter MP, Peters JH, Hagen JA, Bremner CB, DeMeester TR (1997) Duodenogastric reflux potentiates the injurious effects of gastroesophageal reflux. J Gastrointest Surg 1:27–33
Sato T, Miwa K, Sahara H et al (2002) The sequential model of Barrett’s esophagus and adenocarcinoma induced by duodeno-esophageal reflux without exogenous carcinogens. Anticancer Res 22(1A):39–44
Goldstein SR, Yang GY, Curtis SK et al (1997) Development of esophageal metaplasia and adenocarcinoma in a rat surgical model without the use of a carcinogen. Carcinogenesis 18(11):2265–2270
Bonner RF, Emmert-Buck M, Cole K et al (1997) Laser capture microdissection: molecular analysis of tissue. Science 278(5342):1481,1483
Vallbohmer D, Peters JH, Oh D et al (2005) Survivin, a potential biomarker in the development of Barrett’s adenocarcinoma. Surgery 138(4):701–706, discussion 706–707
Levrat M, Lambert R, Kirshbaum G (1962) Esophagitis produced by reflux of duodenal contents in rats. Am J Dig Dis 7(6):564–573
Mirvish SS, Huang Q, Chen SC et al (1993) Metabolism of carcinogenic nitrosamines in the rat and human esophagus and induction of esophageal adenocarcinoma in rats. Endoscopy 25(9):627–631
Clark GW, Smyrk TC, Mirvish SS et al (1994) Effect of gastroduodenal juice and dietary fat on the development of Barrett’s esophagus and esophageal neoplasia: an experimental rat model. Ann Surg Oncol 1(3):252–261
Oberg S, Lord RV, Peters JH et al (2000) Is adenocarcinoma following esophagoduodenostomy without carcinogen in the rat reflux-induced? J Surg Res 91(2):111–117
Pera M, Pera M (2002) Experimental Barrett’s esophagus and the origin of intestinal metaplasia. Chest Surg Clin North Am 12(1):25–37
Kumagai H, Mukaisho K, Sugihara H et al (2003) Cell kinetic study on histogenesis of Barrett’s esophagus using rat reflux model. Scand J Gastroenterol 38(7):687–692
Buskens CJ, Hulscher JBF, van Gulik TM et al (2006) Histopathologic evaluation of an animal model for Barrett’s esophagus and adenocarcinoma of the distal esophagus. J Surg Res 135(2):337–344
Warson C, Van De Bovenkamp JHB, Korteland-Van Male AM et al (2002) Barrett’s esophagus is characterized by expression of gastric-type mucins (MUC5AC, MUC6) and TFF peptides (TFF1 and TFF2), but the risk of carcinoma development may be indicated by the intestinal-type mucin, MUC2. Human Pathol 33(6):660–668
DeMeester SR, DeMeester TR (2000) Columnar mucosa and intestinal metaplasia of the esophagus: fifty years of controversy. Ann Surg 231(3):303–321
Oh DS, Hagen JA, Fein M et al (2006) Impact of reflux composition on mucosal injury and esophageal function. J Gastrointest Surg 10(6):787–797
Campos GM, DeMeester SR, Peters JH et al (2001) Predictive factors of Barrett esophagus: multivariate analysis of 502 patients with gastroesophageal reflux disease. Arch Surg 136(11):1267–1273
Barley NF, Taylor V, Shaw-Smith CJ et al (2003) Human ileal bile acid-binding protein promoter and the effects of CDX2. Biochim Biophys Acta 1630(2–3):138–143
Kazumori H, Ishihara S, Rumi MAK et al (2006) Bile acids directly augment caudal related homeobox gene Cdx2 expression in oesophageal keratinocytes in Barrett’s epithelium. Gut 55(1):16–25
Hu Y, Williams VA, Gellersen O et al (2007) The pathogenesis of Barrett’s esophagus: secondary bile acids upregulate intestinal differentiation factor CDX2 expression in esophageal cells. J Gastrointest Surg 11(7):827–834
Hu Y, Jones C, Gellersen O et al (2007) Pathogenesis of Barrett esophagus: deoxycholic acid up-regulates goblet-specific gene MUC2 in concert with CDX2 in human esophageal cells. Arch Surg 142(6):540–544, discussion 544–545
Shimada H, Koike T, Yamagata M, Yoneda M, Hiraishi H (2007) Regulation of TFF3 expression by homeodomain protein CDX2. Regulat Peptides 140:81–87
Baus-Loncar M, Giraud AS (2005) Multiple regulatory pathways for trefoil factor (TFF) genes. Cell Molec Life Sci 62(24):2921–2931
Emami S, Rodrigues S, Rodrigue CM et al (2004) Trefoil factor family (TFF) peptides and cancer progression. Peptides 25(5):885–898
Acknowledgement
This study was funded by a SAGES Research Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oh, D.S., DeMeester, S.R., Dunst, C.M. et al. Validation of a rodent model of Barrett’s esophagus using quantitative gene expression profiling. Surg Endosc 23, 1346–1352 (2009). https://doi.org/10.1007/s00464-008-0169-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-008-0169-5