Abstract
Background
Several studies have shown that laparoscopic surgery (LS) minimizes surgical trauma and the immune function is better preserved. Another major advantage of LS is the lower incidence of septic complications. However, several in vitro studies have shown that CO2 severely impairs macrophage physiology. In theory, this would reduce the ability to respond to peritoneal contamination. However, there is some controversy in view of the evidence of a better preserved peritoneal response to sepsis. This study analyzed the early response of the peritoneum to contamination in a CO2 ambience.
Methods
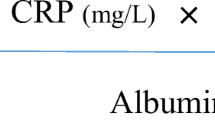
A total of 192 CD-1 mice were distributed in three groups: group 1, laparotomy (LAP, n = 64); group 2, CO2 laparoscopy (CO2-LC, n = 64); and group 3, wall lift laparoscopy (WL-LC, n = 64). Mice in each group were randomized to receive 1 ml of Escherichia coli suspension (1 × 104 colony-forming units/ml) or saline. Peritoneal fluid was obtained at 1.5, 3, 6, and 12 h after surgery. Monocyte chemoattractant protein-1 (MCP-1), interleukin-6 (IL-6), and prostaglandin E2 (PGE2) were measured.
Results
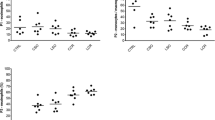
MCP-1 levels were significantly greater and higher earlier in group 2 (CO2-LC) than in group 1 (LAP) (p < 0.007). Simultaneously, the increment in the traction group (WL-LC, group 3) was significantly higher (p < 0.002) than after laparotomy, with no differences in group 2 (CO2-LC). When a contamination was added to the laparotomy subgroup, there was a significant increase compared to the group without contamination (p < 0.5). MCP-1 modifications after contamination in the LAP group were statistically significant and appeared later than in the WL-LC (p < 0.002) and CO2-LC groups (p < 0.02). For IL-6, the three models presented a significant increase in the noncontaminated groups. This occurred significantly later in the LAP group. Simultaneously, the increase in IL-6 occurred earlier and was significantly higher in the WL-LC group compared to the LAP group (p < 0.003), without differences between CO2-LC and wall lift groups. Significant differences between contaminated and noncontaminated subgroups were only observed in the LC-CO2 groups. When contaminated, the traction model sustained a higher and earlier rise in IL-6 levels compared to the LAP and LC-CO2 groups (p < 0.001). For PGE2, The three models showed a significant increase in PGE2 levels in the noncontaminated groups. However, there were no significant differences between them. In the contaminated groups, there was no statistical difference between the groups.
Conclusion
Despite a transient impairment of the immediate peritoneal response to a septic challenge, the degree of injury with LS is lower than that with open surgery, and abdominal infection can therefore be better controlled.




Similar content being viewed by others
References
Are C, Talamini MA, Murata K, De Maio A (2002) Carbon dioxide pneumoperitoneum alters acute-phase response induced by lipopolysaccharide. Surg Endosc 16: 1464–1467
Bachman SL, Hanly EJ, Nwanko JI, Lamb J, Herring AE, Marohn MR, De Maio A, Talamini MA (2004) The effect of timing of pneumoperitoneum on the inflammatory response. Surg Endosc, in press
Balague C, Targarona EM, Pujol M, Filella X, Espert JJ, Trias M (1999) Peritoneal response to a septic challenge. Comparison between open laparotomy, pneumoperitoneum laparoscopy, and wall lift laparoscopy. Surg Endosc 13: 792–796
Bentes de Souza AM, Wang CC, Chu CY, Lam PM, Rogers MS (2003) The effect of intra-abdominal pressure on the generation of 8-iso prostaglandin F2alpha during laparoscopy in rabbits. Hum Reprod 18: 2181–2188
Bloechle C, Kluth D, Holstein AF, Emmermann A, Strate T, Zornig C, Izbicki JR (1999) A pneumoperitoneum perpetuates severe damage to the ultrastructural integrity of parietal peritoneum in gastric perforation-induced peritonitis in rats. Surg Endosc 13: 683–688
Brodsky JA, Brody FJ, Endlich B, Amstrong DA, Ponsky JL, Hamilton IA (2002) MCP-1 is highly expressed in peritoneum following midline laparotomy with peritoneal abrasion in a murine model. Surg Endosc 16: 1079–1082
Buunen M, Gholghesaei M, Veldkamp R, Meijer DW, Bonjer HJ, Bouvy ND (2004) Stress response to laparoscopic surgery: a review. Surg Endosc 18: 1022–1028 DOI: 10.1007/s00464-003-9169-7
Cavaillon JM (1994) Cytokines and macrophages. Biomed Pharmacother 48: 445–453
Hahn EL, He L, Gamelli RL (2000) PGE2 synthesis and metabolism in burn injury and trauma. J Trauma 49: 1147–1154
Hanly EJ, Mendoza-Sagaon M, Murata K, Hardacre JM, De Maio A, Talamini MA (2003) CO2 pneumoperitoneum modifies the inflammatory response to sepsis. Ann Surg 237: 343–350
Hazebroek EJ, Schreve MA, Visser P, De Bruin RW, Marquet RL, Bonjer HJ (2002) Impact of temperature and humidity of carbon dioxide pneumoperitoneum on body temperature and peritoneal morphology. J Laparoendosc Adv Surg Tech A 12: 355–364
Hong SJ, Cho EJ, Lee JY, Kim WW, Kim EK (2003) The physiologic response to laparoscopic cholecystectomy: CO2 pneumoperitoneum vs wall lift. Can J Anaesth 50: 200–201
Ishizuka B, Kuribayashi Y, Kobayashi Y, Hamada N, Abe Y, Amemiya A, Aoki T, Satoh T (2000) Stress responses during laparoscopy with CO2 insufflation and with mechanical elevation of the abdominal wall. J Am Assoc Gynecol Laparosc 7: 363–371
Iwanaka T, Arkovitz MS, Arya G, Ziegler M (1997) Evaluation of operative stress and peritoneal macrophage function in minimally invasive operations. J Am Coll Surg 184: 357–363
Japanese Association of Abdominal Wall Lifting for vs Laparoscopic Surgery (1999) Comparison between CO2 insufflation and abdominal wall lift in laparoscopic cholecystectomy. Surg Endosc 13: 705–709
Kalff JC, Turler A, Schwarz NT, Schraut WH, Lee KK, Tweardy DJ, Billiar TR, Simmons RL, Bauer AJ (2003) Intra-abdominal activation of a local inflammatory response within the human muscularis externa during laparotomy. Ann Surg 237: 301–315
Kopernik G, Avinoach E, Grossman Y, Levy R, Yulzari R, Rogachev B, Douvdevani A (1998) The effect of high partial pressure of CO2 environment on human peritoneal cells. Relevance to carbon dioxide pneumoperitoneum. Am J Obstet Gynecol 179: 1503–1510
Kuntz C, Wunsch A, Bodeker C, Bay F, Rosch R, Windeler J, Herfarth C (2000) Effect of pressure and gas type on intraabdominal, subcutaneous and blood pH in laparoscopy. Surg Endosc 14: 367–371
Luster AD (1998) Chemokines—chemotactic cytokines that mediate inflammation. Lancet 338: 436–445
Matsumoto T, Dolgor B, Ninomiya K, Bandoh T, Yoshida T, Kitano S (2001) Effect of CO2 pneumoperitoneum on the systemic and peritoneal cytokine response in a LPS-induced sepsis model. Eur Surg Res 33: 71–76
Matsumoto T, Tsuboi S, Dolgor B, Bandoh T, Yoshida T, Kitano S (2001) The effect of gases in the intraperitoneal space on cytokine response and bacterial translocation in a rat model. Surg Endosc 15: 80–84
Neuhaus SJ, Watson DI (2004) Pneumoperitoneum and peritoneal surface changes: a review. Surg Endosc 18: 1316–1322 [Epub ahead of print]
Neuhaus SJ, Watson DL, Ellis T, Lafullarde T, Jamieson GG, Russell WJ (2001) Metabolic and immunologic consequences of laparoscopy with helium or CO2 insufflation: a randomized clinical trial. ANZ J Surg 71: 447–452
Novitsky YW, Litwin DEM, Callery MP (2004) The net immunologic advantage of laparoscopic surgery. Surg Endosc 18: 1411–1419 DOI: 10.1007/s00464-003-8275x
Puttick MI, Scott DM, Dye J, Nduka CC, Menzies NM, Mansfield AO, Darzi A (1999) Comparison of immunologic and physiologic effects of CO2 pneumoperitoneum at room and body temperatures. Surg Endosc 13: 572–575
Riese J, Niedobitek G, Lisner R, Jung A, Hohenberger W, Haupt W (2004) Expression of interleukin-6 and monocyte chemoattractant protein-1 by peritoneal sub-mesothelial cells during abdominal operations. J Pathol 202: 34–40
Riese J, Schoolmann S, Denzel C, Herrmann O, Hohenberger W, Haupt W (2002) Effect of abdominal infections on peritoneal and systemic production of interleukin 6 and monocyte chemoattractant protein-1. Shock 17: 361–364
Romeo C, Impellizzeri P, Antonuccio P, et al. (2003) Peritoneal macrophage activity after laparoscopy or laparotomy. J Pediatr Surg 38: 97–101.
Rotstein OD (2001) Peritoneal host defences: modulation by CO2 insufflation. Surg Infect 2: 163–168
Sendt W, Amberg R, Schoffel U, Hassan A, von Specht BU, Farthmann EH (1999) Local inflammatory peritoneal response to operative trauma: studies on cell activity, cytokine expression, and adhesion molecules. Eur J Surg 165: 1024–1030
Sido B, Teklote JR, Hartel M, Friess H, Buchler MW (2004) Inflammatory response after abdominal surgery. Best Pract Res Clin Anaesth 18: 439–454
Sietses C, Blomberg ME, Eijsbouts QAJ, Beelen RHJ, Berends FJ, Cuesta MA (2002) The influence of CO2 vs helium insufflation or the abdominal wall lifting technique on the systemic immune response. Surg Endosc 16: 525–528
Suematsu T, Hirabayashi Y, Shiraishi N, Adachi Y, Kitamura H, Kitano S (2001) Morphology of the murine peritoneum after pneumoperitoneum vs laparotomy. Surg Endosc 15: 954–958
Sylla P, Kirman I, Whelan RL (2005) Immunological advantages of advanced laparoscopy. Surg Clin North Am 85: 1–8
Targarona EM, Balague C, Knook MM, Trias M (2000) Laparoscopic surgery and surgical infection. Br J Surg 87: 536–544
Targarona EM, Pons MJ, Balagué C, et al. (1996) Acute phase is the only significantly reduced component of the injury response after laparoscopic cholecystectomy. World J Surg 20: 528–533
Topley N, Petersen MM, Mackenzie R, et al. (1994) Human peritoneal mesothelial cell prostaglandins synthesis: induction of cyclooxygenase mRNA by peritoneal macrophage-derived cytokines. Kidney Int. 46: 900–909
Ure BM, Niewold TA, Bax NM, Ham M, van der Zee DC, Essen GJ (2002) Peritoneal, systemic, and distant organ inflammatory responses are reduced by a laparoscopic approach and carbon dioxide versus air. Surg Endosc 16: 836–842
van den Tol PM, van Rossen EE, van Eijck CH, Bonthuis F, Marquet RL, Jeekel H (1998) Reduction of peritoneal trauma by using nonsurgical gauze leads to less implantation metastasis of spilled tumor cells. Ann Surg 227: 242–248
Volz J, Koster S, Spacek Z, Paweletz N (1999) Characteristics alteration of the peritoneum after carbon dioxide pneumoperitoneum. Surg Endosc 13: 611–614
West MA, Hackman DJ, Baker J, Rodriguez JL, Bellingham J, Rotstein OD (1997) Mechanism of decreased in vitro murine macrophage cytokine release after exposure to carbon dioxide. Relevance to laparoscopic surgery. Ann Surg 226: 179–190
Wildbrett P, Oh A, Naundorf D, Volk T, Jacobi CA (2003) Impact of laparoscopic gases on peritoneal microenvironment and essential parameters of cell function. Surg Endosc 17: 78–82
Acknowledgment
This work was supported by FIS grant 99/0341.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Targarona, E.M., Rodríguez, M., Camacho, M. et al. Immediate peritoneal response to bacterial contamination during laparoscopic surgery. Surg Endosc 20, 316–321 (2006). https://doi.org/10.1007/s00464-005-0367-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-005-0367-3