Abstract
Background
The immunologic repercussions due to cavity insufflation are the focus of great discussion. The aim of this study was to compare the inflammatory response and bacterial dissemination after laparotomy and abdominal CO2 insufflation in a murine model of peritonitis.
Methods
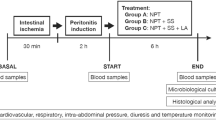
Swiss mice were inoculated intraperitoneally with 0.5 ml of a solution containing 1 × 108 colony-forming units (CFU)/ml of Escherichia coli and were divided into three groups as follow: control (anesthesia for 30 min), laparotomy (2.5-cm midline incision for 30 min), and CO2 pneumoperitoneum (CO2 cavity insufflation for 30 min). The number of leukocytes, CFU/ml counting, and the levels of interleukin (IL)-6, tumor necrosis factor-α (TNF-α), and IL-10 were evaluated in blood, peritoneal, and pleural fluid samples obtained at 90 min and 18 h after the procedures.
Results
The laparotomy group showed a greater bacterial dissemination to the blood, peritoneum, and pleural cavity and also greater neutrophil migration to the peritoneal cavity compared to the CO2 insufflated and control groups. The 24-h mortality was also significantly higher in the laparotomy group. The IL-6 levels showed a precocious rise in all groups submitted to bacterial inoculation at the 90-min time point. At the 18-h time point, IL-6 levels in the peritoneum were significantly higher in the laparotomy group than in the control or CO2 insufflated groups. At the same time, TNF-α levels were higher in the laparotomy and CO2 insufflated groups than in controls; IL-10 levels showed no differences among the groups.
Conclusions
Our results suggest that cavity insufflation with CO2 is a more effective method of access, inducing less bacterial dissemination and also a less intense inflammatory response. Cavity insufflation with CO2 may present a good option for the surgical treatment of patients with bacterial peritonitis.







Similar content being viewed by others
References
Balagué C, Targarona EM, Pujol M, Filella X, Espert JJ, Trias M (1999) Peritoneal response to a septic challenge. Surg Endoscosc 13: 792–796
Bone RC (1996) Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med 24: 1125–1127
Delogu G, Famularo G, Moretti S, De Luca A, Tellan G, Antonucci A, Marandola M, Signore L (2001) Interleukin-10 and apoptotic death of circulating lymphocytes in surgical/anesthesia trauma. J trauma 51: 92–97
de Waal Malefyt R, Abrams R, Benett B, Figdor CG, Vries JE (1991) Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 174: 1209–1220
Gurtner GC, Robertson CS, Chung SC, Ling TK, Ip SM, Li AK (1995) Effect of carbon dioxide pneumoperitoneum on bacteremia and endotoxemia in an animal model of peritonitis. Br J Surg 82: 844–848
Hajri A, Mutter D, Wack S, Bastien C, Gury JF, Marescaux J, Aprahamian M (2000) Dual affect of laparoscopy on cell-mediated immunity. Eur Surg Res 32: 261–266
Hanly EJ, Mendoza-Sagaon M, Murata K, Hardacre JM, Demaio A, Talamini MA (2003) CO2 pneumoperitoneum modifies the inflammatory response to sepsis. Ann Surg 237: 343–350
Ipek T, Paksoy M, Colak T, Polat E, Uygun N (1998) Effect of carbon dioxide pneumoperitoneum on bacteremia and severity of peritonitis in an experimental model. Surg Endosc 12: 432–435
Kawasaki T, Ogata M, Kawasaki C, Tomihisa T, Okamoto K, Shigematsu A (2001) Surgical stress induces endotoxin hyporresponsiveness and an early decrease of monocyte CD14 and HLA-DR expression during surgery. Anesth analg 92: 1322–1326
Majetschak M, Flach R, Kreuzfelder E, Jennissen V, Heukamp T, Neudeck F, Schmit-Neurburg KP, Obertacke U, Schade FU (1999) The extend of traumatic damage determines a graded depression of the endotoxin responsiveness of peripheral blood mononuclear cells from patients with blunt injuries. Crit Care Med 27: 313–318
Matsumoto T, Dolgor B, Ninomiya K, Bandoh T, Yoshida T, Kitano S (2001) Effect of CO2 pneumoperitoneum on the systemic and peritoneal cytokine response in a LPS-induced sepsis model. Eur Surg Res 33: 71–76
Mehigan BJ, Hartley JE, Drew PJ, Saleh A, Dore PC, Lee PW, Monson JRT (2001) Changes in T cell sets, interleukin-6, and C-reactive protein after laparoscopic and open colorectal resection for malignancy. Surg Endosc 15: 1289–1293
Ogata M, Okamoto K, Kzuari K, Kawasaki T, Itoh I, Shigematsu A (2000) Role of interleukin-10 on hyporesponsiveness of endotoxin during surgery. Crit Care Med 28: 3166–3169
Özmen MM, Col C, Aksoy AM, Tekeli FA, Berberoglu M (1999) Effect of CO2 insufflations on bacteremia and bacterial translocation in an animal model of peritonitis. Surg Endosc 13: 801
Pross M, Mantke R, Kuns D, Reinheckel T, Halangk W, Lippert H, Schulz HV (2002) Reduced neutrophil sequestration in lung tissue after laparoscopic lavage in a rat peritonitis model. World J Surg 26: 49–53
Redmond HP, Watson WG, Houghton T, Condron C, Watson RGK, Bouchier-Hayes DB (1994) Immune function in patients undergoing open vs laparoscopic cholecystectomy. Arch Surg 129: 1240–1246
Rongione AF, Kusske AM, Kwan K, Ashley SW, Reber HA, McFadden DW (2000) Interleukin-10 protects against lethality of intra-abdominal infection and sepsis. J Gastrointest Surg 4: 70–76
Sanna A, Adani GL, Donini A (2003) The role of laparoscopy in patients with suspected peritonitis: experience of a single institution. Adv Surg Tech A 13: 17–19
Schwenk W, Jacobi C, Mansmann U, Bohm B, Muller JM (2000) Inflammatory response after laparoscopic and conventional colorectal resections—results of a prospective randomized trial. Langenbecks Arch Surg 385: 2–9
Sietses C, Havenith CEG, Eijsbouts QAJ, van Leeuwen PAM, Meijer S, Beelen RHJ, Cuesta MA (2000) Laparoscopic surgery preserves monocyte-mediated tumor cell killing in contrast to the conventional approach. Surg Endosc 14: 456–460
Sietses C, Wieser MJ, Eijsbouts QA, van Leelwen PA, Beelen RH, Meijer S, Cuesta MA (2000) The influence of laparoscopic surgery on postoperative polymorphonuclear leukocyte function. Surg Endosc 14: 812–816
Siu WT, Leong HT, Law BK, Chau CH, Li AC, Fung KH, Tay YP, Li MK (2002) Laparoscopic repair for perforated peptic ulcer: a randomized controlled trial. Ann Surg 235: 313–319
Suematsu T, Hirabayashi Y, Shiraishi N, Adachi Y, Kitamura H, Kitano S (2001) Morphology of the murine peritoneum after pneumoperitoneum vs laparotomy. Surg Endosc 15: 954–958
Targarona EM, Balagué C, Trías M (2000) Laparoscopic surgery and surgical infection. Br J Surg 87: 536–544
Ure BM, Niewold TA, Bax NMA, Ham M, van Der Zee CC, Essen GJ (2002) Peritoneal, systemic, and distant organ inflammatory responses are reduced by a laparoscopic approach and carbon dioxide vs air. Surg Endosc 16: 836–842
van Berge Henegouwen MI, van der Poll T, van Deventer SJ, Gouma DJ (1998) Peritoneal cytokine release after elective gastrointestinal surgery and postoperative complications. Am J Surg 175: 311–316
Vianna RC, Gomes RN, Bozza FA, Amancio RT, Bozza PT, David CM, Castro-Faria-Neto HC (2004) Antibiotic treatment in a murine model of sepsis: impact on cytokines and endotoxin release. Shock 21: 115–120
Wakefield CH, Carey D, Foulds S, Monson JRT, Guillou PJ (1993) Polymorphonuclear leukocyte activation. An early marker of postsurgical sepsis response. Arch Surg 128: 390–395
Watson RW, Redmond HP, McCarthy J, Burk PE, Bouchier-Hayes D (1995) Exposure of the peritoneal cavity to air regulates early inflammatory responses to surgery in a murine model. Br J Surg 82: 1060–1065
West MA, Hackmam DJ, Baker J, Rodiguez JL, Bellingham J, Rotstein OD (1997) Mechanism of decreased in vitro murine macrophage cytokine release after exposure to carbon dioxide. Ann Surg 226: 179–190
Zellweger R, Ayala A, Zhu XL, Morrison MH, Chaudry IH (1995) Effect of surgical trauma on splenocite and peritoneal macrophage immune function. J Trauma 39: 645–650
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pitombo, M.B., Lupi, O.H., Gomes, R.N. et al. Inflammatory response and bacterial dissemination afterlaparotomy and abdominal CO2 insufflation in a murine model of peritonitis. Surg Endosc 20, 1440–1447 (2006). https://doi.org/10.1007/s00464-005-0117-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-005-0117-6