Abstract
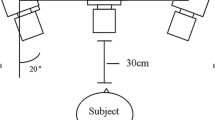
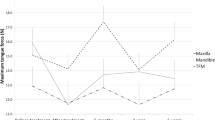
Surgical resection in oral cancer patients can result in altered speech, swallowing, and patient perception of quality of life (QOL). Oral surgery can result in reduced lingual range of motion (ROM). However, few studies have quantified the degree of lingual restriction after surgery. This pilot study describes a new measurement system to define tongue ROM in surgically treated tongue cancer patients. This measurement system was validated by comparing results in these treated surgical patients versus healthy individuals. This scale was further validated by correlating ROM with performance status, oral outcomes, and patient-rated QOL. Thirty-six patients who underwent oral tongue surgery and 31 healthy individuals were included. Tongue ROM was assessed using a novel ROM assessment system. This novel system was examined in these patients versus healthy subjects. This measurement tool was further validated by correlating tongue ROM in treated patients with performance status, oral outcomes, and patient-rated QOL. Tongue ROM was found to be significantly lower in the surgically treated patients than in the healthy individuals (p = 0.0001). Tongue ROM correlated with performance status, oral outcomes, and all QOL measures. This new tongue ROM measurement system defined tongue deficits in surgically treated oral cancer patients. This tool was validated by comparing results to those in healthy individuals, as well as by correlating tongue ROM to performance status, oral outcomes, and QOL. This measurement tool can be used to define baseline and postsurgery tongue ROM in oral cancer patients, as well as track change over time with recovery and therapy. Future studies should examine use of this measurement tool with other populations demonstrating tongue deficits.





Similar content being viewed by others
References
Pauloski BR, et al. Speech and swallowing function after anterior tongue and floor of mouth resection with distal flap reconstruction. J Speech Hear Res. 1993;36(2):267–76.
Pauloski BR, et al. Speech and swallowing function after oral and oropharyngeal resections: one-year follow-up. Head Neck. 1994;16(4):313–22.
Pauloski BR, Logemann JA. Impact of tongue base and posterior pharyngeal wall biomechanics on pharyngeal clearance in irradiated postsurgical oral and oropharyngeal cancer patients. Head Neck. 2000;22(2):120–31.
Pauloski BR, et al. Surgical variables affecting speech in treated patients with oral and oropharyngeal cancer. Laryngoscope. 1998;108(6):908–16.
Furia CL, et al. Speech intelligibility after glossectomy and speech rehabilitation. Arch Otolaryngol Head Neck Surg. 2001;127(7):877–83.
Rentschler GJ, Mann MB. The effects of glossectomy on intelligibility of speech and oral perceptual discrimination. J Oral Surg. 1980;38(5):348–54.
Biazevic MG, et al. Survival and quality of life of patients with oral and oropharyngeal cancer at 1-year follow-up of tumor resection. J Appl Oral Sci. 2010;18(3):279–84.
Dwivedi RC, et al. An exploratory study of the influence of clinico-demographic variables on swallowing and swallowing-related quality of life in a cohort of oral and oropharyngeal cancer patients treated with primary surgery. Eur Arch Otorhinolaryngol. 2012;269(4):1233–9.
Yang ZH, et al. Quality of life of patients with tongue cancer 1 year after surgery. J Oral Maxillofac Surg. 2010;68(9):2164–8.
McConnel FM, et al. Functional results of primary closure vs flaps in oropharyngeal reconstruction: a prospective study of speech and swallowing. Arch Otolaryngol Head Neck Surg. 1998;124(6):625–30.
Suarez-Cunqueiro MM, et al. Speech and swallowing impairment after treatment for oral and oropharyngeal cancer. Arch Otolaryngol Head Neck Surg. 2008;134(12):1299–304.
Hanasono MM, et al. Impact of reconstructive microsurgery in patients with advanced oral cavity cancers. Head Neck. 2009;31(10):1289–96.
Urken ML, et al. A systematic approach to functional reconstruction of the oral cavity following partial and total glossectomy. Arch Otolaryngol Head Neck Surg. 1994;120(6):589–601.
Brown L, et al. A longitudinal study of functional outcomes after surgical resection and microvascular reconstruction for oral cancer: tongue mobility and swallowing function. J Oral Maxillofac Surg. 2010;68(11):2690–700.
Seikaly H, et al. Functional outcomes after primary oropharyngeal cancer resection and reconstruction with the radial forearm free flap. Laryngoscope. 2003;113(5):897–904.
Rieger JM, et al. Functional outcomes after surgical reconstruction of the base of tongue using the radial forearm free flap in patients with oropharyngeal carcinoma. Head Neck. 2007;29(11):1024–32.
Dodds WJ, Stewart ET, Logemann JA. Physiology and radiology of the normal oral and pharyngeal phases of swallowing. AJR Am J Roentgenol. 1990;154(5):953–63.
Shin YS, et al. Radiotherapy deteriorates postoperative functional outcome after partial glossectomy with free flap reconstruction. J Oral Maxillofac Surg. 2012;70(1):216–20.
Michiwaki Y, et al. Functional effects of intraoral reconstruction with a free radial forearm flap. J Craniomaxillofac Surg. 1990;18(4):164–8.
Imai S, Michi K. Articulatory function after resection of the tongue and floor of the mouth: palatometric and perceptual evaluation. J Speech Hear Res. 1992;35(1):68–78.
Chepeha DB, et al. Rectangle tongue template for reconstruction of the hemiglossectomy defect. Arch Otolaryngol Head Neck Surg. 2008;134(9):993–8.
Michi K, et al. Improvement of speech intelligibility by a secondary operation to mobilize the tongue after glossectomy. J Craniomaxillofac Surg. 1989;17(4):162–6.
Leder SB, et al. Can an oral mechanism examination contribute to the assessment of odds of aspiration? Dysphagia. 2013;28(3):370–4.
Husaini H, et al. Survey of functional assessments used by speech-language pathologists for oral cancer patients. Dysphagia. doi:10.1007/s00455-014-9520-2.
List MA, et al. Quality of life and performance in advanced head and neck cancer patients on concomitant chemoradiotherapy: a prospective examination. J Clin Oncol. 1999;17(3):1020–8.
List MA, Ritter-Sterr C, Lansky SB. A performance status scale for head and neck cancer patients. Cancer. 1990;66(3):564–9.
Schag C, Heinrich R, Ganz P. Karnofsky performance status scale revisited: reliability, validity and guidelines. J Clin Oncol. 1984;2:187–93.
Belafsky PC, et al. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann Otol Rhinol Laryngol. 2008;117(12):919–24.
Chen AY, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127(7):870–6.
Dwivedi RC, et al. First report on the reliability and validity of speech handicap index in native English-speaking patients with head and neck cancer. Head Neck. 2011;33(3):341–8.
Rinkel RN, et al. Speech Handicap Index in patients with oral and pharyngeal cancer: better understanding of patients’ complaints. Head Neck. 2008;30(7):868–74.
Lazarus CL, et al. Tongue strength as a predictor of functional outcomes and quality of life after tongue cancer surgery. Ann Otol Rhinol Laryngol. 2013;122(6):386–97.
Lazarus CL, et al. Swallowing and tongue function following treatment for oral and oropharyngeal cancer. J Speech Lang Hear Res. 2000;43(4):1011–23.
Magne P, Gallucci GO, Belser UC. Anatomic crown width/length ratios of unworn and worn maxillary teeth in white subjects. J Prosthet Dent. 2003;89(5):453–61.
Connor NP, et al. Impact of conventional radiotherapy on health-related quality of life and critical functions of the head and neck. Int J Radiat Oncol Biol Phys. 2006;65(4):1051–62.
Kohler PF, Winter ME. A quantitative test for xerostomia. The Saxon test, an oral equivalent of the Schirmer test. Arthritis Rheum. 1985;28(10):1128–32.
Urken ML, Okay DJ. Reconstruction of the oral cavity following partial and total glossectomy. In: Urken ML, editor. Multidisciplinary head & neck reconstruction: a defect-oriented approach. New York: Wolters Kluwer/Lippincott Williams & Wilkins; 2010. p. 235–320.
Nicosia MA, et al. Age effects on the temporal evolution of isometric and swallowing pressure. J Gerontol A Biol Sci Med Sci. 2000;55(11):M634–40.
Weber C, et al. Limited mouth opening after primary therapy of head and neck cancer. Oral Maxillofac Surg. 2010;14(3):169–73.
Navazesh M, Christensen CM. A comparison of whole mouth resting and stimulated salivary measurement procedures. J Dent Res. 1982;61(10):1158–62.
Chen PH, et al. Prevalence of perceived dysphagia and quality-of-life impairment in a geriatric population. Dysphagia. 2009;24(1):1–6.
Furia CL, et al. Video fluoroscopic evaluation after glossectomy. Arch Otolaryngol Head Neck Surg. 2000;126(3):378–83.
Logemann JA et al. Speech and swallowing rehabilitation for head and neck cancer patients. Oncology (Williston Park), 1997;11(5):651–6, 659; discussion 659, 663–4.
Lazarus C. Effects of radiotherapy on tongue strength in head and neck cancer patients. In: American Speech-Language-Hearing Association Annual Meeting 2005, San Diego, CA.
Pauloski BR, et al. Speech and swallowing in irradiated and nonirradiated postsurgical oral cancer patients. Otolaryngol Head Neck Surg. 1998;118(5):616–24.
McConnel FM, et al. Surgical variables affecting postoperative swallowing efficiency in oral cancer patients: a pilot study. Laryngoscope. 1994;104(1 Pt 1):87–90.
Zelefsky MJ et al. Long-term subjective functional outcome of surgery plus postoperative radiotherapy for advanced stage oral cavity and oropharyngeal carcinoma. Am J Surg. 1996;171(2):258–61; discussion 262.
Teguh DN, et al. Treatment techniques and site considerations regarding dysphagia-related quality of life in cancer of the oropharynx and nasopharynx. Int J Radiat Oncol Biol Phys. 2008;72(4):1119–27.
Acknowledgments
Thanks go to Sebastien Delice for his contribution of data input and to Eileen Stevens for her contributions with data accrual.
Conflict of interest
No authors have any conflict of interest or disclosures to acknowledge.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lazarus, C.L., Husaini, H., Jacobson, A.S. et al. Development of a New Lingual Range-of-Motion Assessment Scale: Normative Data in Surgically Treated Oral Cancer Patients. Dysphagia 29, 489–499 (2014). https://doi.org/10.1007/s00455-014-9534-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00455-014-9534-9