Abstract
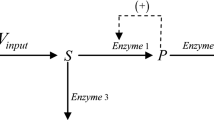
The multiple steady states in an isothermal, constant-density CSTR involving two-substrates, enzyme- catalyzed reactions is determined by a zero eigenvalue analysis. The hysteresis and bistability occurs for a certain range of the rate constant of product formation from a ternary complex, k ES1S2→P+E . A two-parameter (k ES1S2→P+E , k 0→S1 ) bifurcation diagram for several different values of flow rate k S1→0 is also presented. It shows that, to maintain the existence of the steady state multiplicity under a fixed flow rate, the larger the rate constant k ES1S2→P+E is, the larger the feed concentration of a substrate is required and the wider the range of that exists. To maintain the existence of the steady state multiplicity for a lower flow rate, it is required to reduce the feed concentration of substrates.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 5 July 1999
Rights and permissions
About this article
Cite this article
Ho, PY., Li, HY. Determination of multiple steady states in an enzyme kinetics involving two substrates in a CSTR. Bioprocess Engineering 22, 557–561 (2000). https://doi.org/10.1007/s004499900111
Issue Date:
DOI: https://doi.org/10.1007/s004499900111