Abstract
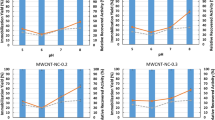
This study aimed to immobilize trypsin on activated carbon submitted to different surface modifications and its application in casein hydrolysis. With the aim of determining which support can promote better maintenance of the immobilized enzyme. Results showed that pH 5.0 was obtained as optimal for immobilization and pH 9.0 for the casein hydrolysis reaction for activated carbon and glutaraldehyde functionalized carbon. Among the supports used, activated carbon modified with iron ions in the presence of a chelating agent was the one that showed best results, under the conditions evaluated in this study. Presenting an immobilization yield of 95.15% and a hydrolytic activity of 4.11 U, same as soluble enzyme (3.76 U). This derivative kept its activity stable at temperatures above 40 °C for1 h and when stored for 30 days at 5 °C. Furthermore, it was effective for more than 6 reuse cycles (under the same conditions as the 1st cycle). In general, immobilization of trypsin on metallized activated carbon can be an alternative to biocatalysis, highlighting the advantages of protease immobilization.




Similar content being viewed by others
References
Liu J, Liu Y, Jin D, Meng M, Jiang Y, Ni L, Liu Z (2019) Immobilization of trypsin onto large-pore mesoporous silica and optimization enzyme activity via response surface methodology. Solid State Sci 89:15–24. https://doi.org/10.1016/j.solidstatesciences.2018.12.014
Mahmood MS, Asghar H, Riaz S, Shaukat I, Zeeshan N, Gul R, Ashraf NM, Saleem M (2022) Expression and immobilization of trypsin-like domain of serine protease from Pseudomonas aeruginosa for improved stability and catalytic activity. Proteins: Struct Funct Bioinf 90:1425–1433. https://doi.org/10.1002/prot.26323
Aggarwal S, Ikram S (2022) Zinc oxide nanoparticles-impregnated chitosan surfaces for covalent immobilization of trypsin: stability & kinetic studies. Int J Biol Macromol 207:205–221. https://doi.org/10.1016/j.ijbiomac.2022.03.014
Dornelles LP, de Souza MDFD, da Silva PM, Procópio TF, Filho RSR, de Albuquerque Lima T, de Oliveira APS, Zingali RB, Paiva PMG, Pontual EV, Napoleão TH (2018) Purification and characterization of a protease from the visceral mass of Mytella charruana and its evaluation to obtain antimicrobial peptides. Food Chem 245:1169–1175. https://doi.org/10.1016/j.foodchem.2017.11.044
Souza Júnior EC, Santos MPF, Sampaio VS, Ferrão SPB, Fontan RCI, Bonomo RCF, Veloso CM (2020) Hydrolysis of casein from different sources by immobilized trypsin on biochar: effect of immobilization method. J Chromatogr B Analyt Technol Biomed Life Sci 1146:122124. https://doi.org/10.1016/j.jchromb.2020.122124
Yamada Y, Obuchi K, Kikuchi N, Almarasy AA, Fujimori A (2022) Immobilization of trypsin from the subphase to the langmuir monolayer of fluorocarbon-modified single-walled carbon nanotube and its activity maintenance. Langmuir 38:5692–5701. https://doi.org/10.1021/acs.langmuir.2c00283
Zhang Y, Zhang Y, Zhang Y, Liang Q, Zhang C, Zhang J, Du G, Du G, Kang Z, Kang Z (2020) Improving production of Streptomyces griseus trypsin for enzymatic processing of insulin precursor. Microb Cell Fact. https://doi.org/10.1186/s12934-020-01338-9
Tavano OL, Berenguer-Murcia A, Secundo F, Fernandez-Lafuente R (2018) Biotechnological applications of proteases in food technology. Compr Rev Food Sci Food Saf 17:412–436. https://doi.org/10.1111/1541-4337.12326
Moran ET (2016) Gastric digestion of protein through pancreozyme action optimizes intestinal forms for absorption, mucin formation and villus integrity. Anim Feed Sci Technol 221:284–303. https://doi.org/10.1016/j.anifeedsci.2016.05.015
Demirkan E, Avci T, Aykut Y (2018) Protease immobilization on cellulose monoacetate/chitosan-blended nanofibers. J Ind Text 47:2092–2111. https://doi.org/10.1177/1528083717720205
BaffaJúnior JC, Viana PA, de Rezende ST, de Fátima Ferreira N, Soares VMG (2018) Immobilization of an alpha-galactosidase from debaryomyces hansenni UFV-1 in cellulose film and its application in oligosaccharides hydrolysis. Food Bioprod Process 111:30–36. https://doi.org/10.1016/j.fbp.2018.06.001
Moslemi M, Homaei A, Toiserkani H (2018) Aspartic acid introduce the functional amine groups on the surface of superparamagnetic Fe(OH)3@Fe3O4 nanoparticles for efficient immobilization of Penaeus vannamei protease. Bioprocess Biosyst Eng 41:749–756. https://doi.org/10.1007/s00449-018-1908-1
Nunes YL, de Menezes FL, de Sousa IG, Cavalcante ALG, Cavalcante FTT, da Silva Moreira K, de Oliveira ALB, Mota GF, da Silva Souza JE, de AguiarFalcão IR, Rocha TG, Valério RBR, Fechine PBA, de Souza MCM, dos Santos JCS (2021) Chemical and physical Chitosan modification for designing enzymatic industrial biocatalysts: how to choose the best strategy? Int J Biol Macromol 181:1124–1170. https://doi.org/10.1016/j.ijbiomac.2021.04.004
Maghraby YR, El-Shabasy RM, Ibrahim AH, Azzazy HMES (2023) Enzyme immobilization technologies and industrial applications. ACS Omega 8:5184–5196. https://doi.org/10.1021/acsomega.2c07560
Nguyen HH, Kim M (2017) An overview of techniques in enzyme immobilization, applied science and convergence. Technology 26:157–163. https://doi.org/10.5757/asct.2017.26.6.157
Cavalcante ALG, Chaves AV, Fechine PBA, Holanda Alexandre JYN, Freire TM, Davi DMB, Neto FS, de Sousa IG, da Silva Moreira K, de Oliveira ALB, de Mattos MC, Oliveira MCF, de Brito MV, Ballereau S, Bernardes-Génisson V, da Fonseca AM, dos Santos JCS (2022) Chemical modification of clay nanocomposites for the improvement of the catalytic properties of Lipase A from Candida antarctica. Process Biochem 120:1–14. https://doi.org/10.1016/j.procbio.2022.05.020
Santos MPF, Porfírio MCP, Junior ECS, Bonomo RCF, Veloso CM (2022) Pepsin immobilization: influence of carbon support functionalization. Int J Biol Macromol 203:67–79. https://doi.org/10.1016/j.ijbiomac.2022.01.135
Mazorra-Manzano MA, Ramírez-Suarez JC, Yada RY (2018) Plant proteases for bioactive peptides release: a review. Crit Rev Food Sci Nutr 58:2147–2163. https://doi.org/10.1080/10408398.2017.1308312
Bamdad F, Shin SH, Suh JW, Nimalaratne C, Sunwoo H (2017) Anti-inflammatory and antioxidant properties of casein hydrolysate produced using high hydrostatic pressure combined with proteolytic enzymes. Molecules 22:1–16. https://doi.org/10.3390/molecules22040609
Bolivar JM, Woodley JM, Fernandez-Lafuente R (2022) Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem Soc Rev 51:6251–6290. https://doi.org/10.1039/d2cs00083k
Ashkan Z, Hemmati R, Homaei A, Dinari A, Jamlidoost M, Tashakor A (2021) Immobilization of enzymes on nanoinorganic support materials: an update. Int J Biol Macromol 168:708–721. https://doi.org/10.1016/j.ijbiomac.2020.11.127
Zahirinejad S, Hemmati R, Homaei A, Dinari A, Hosseinkhani S, Mohammadi S, Vianello F (2021) Nano-organic supports for enzyme immobilization: scopes and perspectives. Colloids Surf B Biointerfaces 204:111774. https://doi.org/10.1016/J.COLSURFB.2021.111774
Li Y, Ogorzalek TL, Wei S, Zhang X, Yang P, Jasensky J, Brooks CL, Marsh ENG, Chen Z (2018) Effect of immobilization site on the orientation and activity of surface-tethered enzymes. Phys Chem Chem Phys 20:1021–1029. https://doi.org/10.1039/c7cp06063g
Cui C, Chen H, Chen B, Tan T (2017) Genipin cross-linked glucose oxidase and catalase multi-enzyme for gluconic acid synthesis. Appl Biochem Biotechnol 181:526–535. https://doi.org/10.1007/s12010-016-2228-z
Furlani IL, Amaral BS, Oliveira RV, Cass QB (2020) Enzyme immobilization: concepts and effects on proteolysis. Quim Nova 43:463–473. https://doi.org/10.21577/0100-4042.20170525
Okura NS, Sabi GJ, Crivellenti MC, Gomes RAB, Fernandez-Lafuente R, Mendes AA (2020) Improved immobilization of lipase from thermomyces lanuginosus on a new chitosan-based heterofunctional support: mixed ion exchange plus hydrophobic interactions. Int J Biol Macromol 163:550–561. https://doi.org/10.1016/j.ijbiomac.2020.07.021
Pinheiro MP, Monteiro RRC, Silva FFM, Lemos TLG, Fernandez-Lafuente R, Gonçalves LRB, dos Santos JCS (2019) Modulation of lecitase properties via immobilization on differently activated Immobead-350: stabilization and inversion of enantiospecificity. Process Biochem 87:128–137. https://doi.org/10.1016/j.procbio.2019.08.016
Barbosa O, Torres R, Ortiz C, Berenguer-Murcia Á, Rodrigues RC, Fernandez-Lafuente R (2013) Heterofunctional supports in enzyme immobilization: from traditional immobilization protocols to opportunities in tuning enzyme properties. Biomacromol 14:2433–2462. https://doi.org/10.1021/bm400762h
Ramani K, Karthikeyan S, Boopathy R, Kennedy LJ, Mandal AB, Sekaran G (2012) Surface functionalized mesoporous activated carbon for the immobilization of acidic lipase and their application to hydrolysis of waste cooked oil: isotherm and kinetic studies. Process Biochem 47:435–445. https://doi.org/10.1016/j.procbio.2011.11.025
Moreira KDS, Barros de Oliveira AL, de Moura Júnior LS, de Sousa IG, Gama Cavalcante AL, SimãoNeto F, Rodrigues Valério RB, ValérioChaves A, de Sousa Fonseca T, Vieira Cruz DM, Vieira Lima G, de Oliveira GP, de Souza MCM, Almeida Fechine PB, de Mattos MC, da Fonseca AM, dos Santos JCS (2022) Taguchi design-assisted co-immobilization of lipase A and B from Candida antarctica onto chitosan: characterization, kinetic resolution application, and docking studies. Chem Eng Research Des 177:223–244. https://doi.org/10.1016/j.cherd.2021.10.033
Zhao YT, Zhang K, Zeng J, Yin H, Zheng W, Li R, Ding A, Chen S, Liu Y, Wu W, Jing Z (2022) Immobilization on magnetic PVA/SA@Fe3O4 hydrogel beads enhances the activity and stability of neutral protease. Enzyme Microb Technol 157:110017. https://doi.org/10.1016/J.ENZMICTEC.2022.110017
Bezerra CS, De Farias Lemos CMG, De Sousa M, Gonçalves LRB (2015) Enzyme immobilization onto renewable polymeric matrixes: Past, present, and future trends. J Appl Polym Sci 132:42125. https://doi.org/10.1002/app.42125
Altinkaynak C, Tavlasoglu S, Özdemir N, Ocsoy I (2016) A new generation approach in enzyme immobilization: organic-inorganic hybrid nanoflowers with enhanced catalytic activity and stability. Enzyme Microb Technol 93–94:105–112. https://doi.org/10.1016/J.ENZMICTEC.2016.06.011
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Boudrant J, Woodley JM, Fernandez-Lafuente R (2020) Parameters necessary to define an immobilized enzyme preparation. Process Biochem 90:66–80. https://doi.org/10.1016/j.procbio.2019.11.026
Santos MPF, Brito MJP, Junior ECS, Bonomo RCF, Veloso CM (2019) Pepsin immobilization on biochar by adsorption and covalent binding, and its application for hydrolysis of bovine casein. J Chem Technol Biotechnol 94:1982–1990. https://doi.org/10.1002/jctb.5981
Yu ZL, Zeng WC, Zhang WH, Liao XP, Shi B (2014) Effect of ultrasound on the activity and conformation of α-amylase, papain and pepsin. Ultrason Sonochem 21:930–936. https://doi.org/10.1016/j.ultsonch.2013.11.002
Voytek P, Gjessing EC (1971) Studies of an anionic trypsinogen and its active enzyme from porcine pancreas. J Biol Chem 246:508–516. https://doi.org/10.1016/s0021-9258(18)62518-9
Sahin S, Ozmen I (2020) Covalent immobilization of trypsin on polyvinyl alcohol-coated magnetic nanoparticles activated with glutaraldehyde. J Pharm Biomed Anal 184:113195. https://doi.org/10.1016/j.jpba.2020.113195
Zaak H, Peirce S, de Albuquerque TL, Sassi M, Fernandez-Lafuente R (2017) Exploiting the versatility of aminated supports activated with glutaraldehyde to immobilize β-galactosidase from aspergillus oryzae. Catalysts. https://doi.org/10.3390/catal7090250
Pinto Brito MJ, Bauer LC, Flores Santos MP, Santos LS, Ferreira Bonomo RC, da Costa Ilhéu Fontan R, Veloso CM (2020) Lipase immobilization on activated and functionalized carbon for the aroma ester synthesis. Microporous Mesoporous Mater. https://doi.org/10.1016/j.micromeso.2020.110576
Mo H, Qiu J, Yang C, Zang L, Sakai E, Chen J (2020) Porous biochar/chitosan composites for high performance cellulase immobilization by glutaraldehyde. Enzyme Microb Technol 138:109561. https://doi.org/10.1016/j.enzmictec.2020.109561
Sanchez A, Cruz J, Rueda N, Dos Santos JCS, Torres R, Ortiz C, Villalonga R, Fernandez-Lafuente R (2016) Inactivation of immobilized trypsin under dissimilar conditions produces trypsin molecules with different structures. RSC Adv 6:27329–27334. https://doi.org/10.1039/c6ra03627a
Post AE, Arnold B, Weiss J, Hinrichs J (2012) Effect of temperature and pH on the solubility of caseins: environmental influences on the dissociation of α S- and β-casein. J Dairy Sci 95:1603–1616. https://doi.org/10.3168/jds.2011-4641
Seabra IJ, Gil MH (2007) Cotton gauze bandage: a support for protease immobilization for use in biomedical applications, Revista Brasileira de Ciencias Farmaceuticas/Brazilian. J Pharm Sci 43:535–542. https://doi.org/10.1590/S1516-93322007000400006
Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC (2004) Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 37:790–802. https://doi.org/10.2144/04375rv01
Mo H, Qiu J (2020) Preparation of chitosan/magnetic porous biochar as support for cellulase immobilization by using glutaraldehyde. Polymers (Basel) 12:1–14. https://doi.org/10.3390/polym12112672
Flores EEE, Cardoso FD, Siqueira LB, Ricardi NC, Costa TH, Rodrigues RC, Klein MP, Hertz PF (2019) Influence of reaction parameters in the polymerization between genipin and chitosan for enzyme immobilization. Process Biochem. https://doi.org/10.1016/j.procbio.2019.06.001
Zdarta J, Meyer AS, Jesionowski T, Pinelo M (2018) A general overview of support materials for enzyme immobilization: characteristics, properties, practical utility. Catalysts. https://doi.org/10.3390/catal8020092
Khoshnevisan K, Poorakbar E, Baharifar H, Barkhi M (2019) Recent advances of cellulase immobilization onto magnetic nanoparticles: an update review. Magnetochemistry 5:36. https://doi.org/10.3390/magnetochemistry5020036
Klein MP, Hackenhaar CR, Lorenzoni ASG, Rodrigues RC, Costa TMH, Ninow JL, Hertz PF (2016) Chitosan crosslinked with genipin as support matrix for application in food process: Support characterization and β-d-galactosidase immobilization. Carbohydr Polym 137:184–190. https://doi.org/10.1016/j.carbpol.2015.10.069
Liu DM, Chen J, Shi YP (2018) Advances on methods and easy separated support materials for enzymes immobilization, TrAC -. Trends Anal Chem 102:332–342. https://doi.org/10.1016/j.trac.2018.03.011
Sun J, Xu B, Shi Y, Yang L, Le Ma H (2017) Activity and stability of trypsin immobilized onto chitosan magnetic nanoparticles. Adv Mater Sci Eng. https://doi.org/10.1155/2017/1457072
Chang YY, Li H, Sun H (2017) Immobilized Metal affinity chromatography (IMAC) for metalloproteomics and phosphoproteomics. In: Inorganic and organometallic transition metal complexes with biological molecules and living cells, pp 329–353. https://doi.org/10.1016/B978-0-12-803814-7.00009-5
Galvão WS, Pinheiro BB, Golçalves LRB, de Mattos MC, Fonseca TS, Regis T, Zampieri D, dos Santos JCS, Costa LS, Correa MA, Bohn F, Fechine PBA (2018) Novel nanohybrid biocatalyst: application in the kinetic resolution of secondary alcohols. J Mater Sci 53:14121–14137. https://doi.org/10.1007/s10853-018-2641-5
Doğan D, Sezer S, Ulu A, Köytepe S, Ateş B (2021) Preparation and characterization of amino-functionalized zeolite/SiO2 materials for trypsin-chymotrypsin co-immobilization. Catal Lett 151:2463–2477. https://doi.org/10.1007/S10562-021-03636-2
Han W, Yamauchi M, Hasegawa U, Noda M, Fukui K, van der Vlies AJ, Uchiyama S, Uyama H (2015) Pepsin immobilization on an aldehyde-modified polymethacrylate monolith and its application for protein analysis. J Biosci Bioeng 119:505–510. https://doi.org/10.1016/j.jbiosc.2014.10.018
Razzaghi M, Homaei A, Mosaddegh E (2018) Penaeus vannamei protease stabilizing process of ZnS nanoparticles. Int J Biol Macromol 112:509–515. https://doi.org/10.1016/j.ijbiomac.2018.01.173
Siddiqui I, Husain Q, Azam A (2020) Exploring the antioxidant effects of peptides from almond proteins using PAni-Ag-GONC conjugated trypsin by improving enzyme stability & applications. Int J Biol Macromol 158:150–158. https://doi.org/10.1016/j.ijbiomac.2020.04.188
Aslani E, Abri A, Pazhang M (2018) Immobilization of trypsin onto Fe3O4@SiO2 –NH2 and study of its activity and stability. Colloids Surf B Biointerfaces 170:553–562. https://doi.org/10.1016/j.colsurfb.2018.06.022
Sánchez-Ramírez J, Martínez-Hernández JL, Segura-Ceniceros P, López G, Saade H, Medina-Morales MA, Ramos-González R, Aguilar CN, Ilyina A (2017) Cellulases immobilization on chitosan-coated magnetic nanoparticles: application for Agave Atrovirens lignocellulosic biomass hydrolysis. Bioprocess Biosyst Eng 40:9–22. https://doi.org/10.1007/s00449-016-1670-1
AvciDuman Y, Tufan G, Kaya AU (2020) Immobilisation of cellulase on vermiculite and the effects on enzymatic kinetics and thermodynamics. Appl Clay Sci. https://doi.org/10.1016/j.clay.2020.105792
Shojaei F, Homaei A, Taherizadeh MR, Kamrani E (2017) Characterization of biosynthesized chitosan nanoparticles from Penaeus vannamei for the immobilization of P. vannamei protease: an eco-friendly nanobiocatalyst. Int J Food Prop 20:1413–1423. https://doi.org/10.1080/10942912.2017.1345935
Hegedüs I, Vitai M, Jakab M, Nagy E (2020) Study of prepared α-chymotrypsin as enzyme nanoparticles and of biocatalytic membrane reactor. Catalysts 10:1–18. https://doi.org/10.3390/catal10121454
Acknowledgements
The authors acknowledge the financial support provided by UESB and CAPES-Brazil.
Funding
This work was supported financially by the Coordination for the Improvement of Higher Education Personnel–Brazil (CAPES)—Finance Code 001 and n.88887.368355/2019–00 (PNPD). And research grant from UESB (PPGECAL).
Author information
Authors and Affiliations
Contributions
MPFS: conceptualization; methodology; writing—original draft; validation; investigation. MAF: methodology. ECSJ: funding acquisition; resources. RCFB: funding acquisition; resources; supervision; writing—review and editing. CMV: project administration; funding acquisition; resources; supervision; writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santos, M.P.F., Ferreira, M.A., Junior, E.C.S. et al. Functionalized activated carbon as support for trypsin immobilization and its application in casein hydrolysis. Bioprocess Biosyst Eng 46, 1651–1664 (2023). https://doi.org/10.1007/s00449-023-02927-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-023-02927-9