Abstract
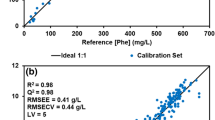
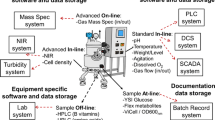
Biologics manufacturing is increasingly moving toward intensified processes that require novel control strategies in order to achieve higher titers in shorter periods of time compared to traditional fed-batch cultures. In order to implement these strategies for intensified processes, continuous process monitoring is often required. To this end, inline Raman spectroscopy was used to develop partial least squares models to monitor changes in residual concentrations of glucose, phenylalanine and methionine during the culture of five different glutamine synthetase piggyBac® Chinese hamster ovary clones cultured using an intensified high inoculation density fed-batch platform process. Continuous monitoring of residual metabolite concentrations facilitated automated feed-rate adjustment of three supplemental feeds to maintain glucose, phenylalanine, and methionine at desired setpoints, while maintaining other nutrient concentrations at acceptable levels across all clones cultured on the high inoculation density platform process. Furthermore, all clones cultured on this process achieved high viable cell concentrations over the course of culture, indicating no detrimental impacts from the proposed feeding strategy. Finally, the automated control strategy sustained cultures inoculated at high cell densities to achieve product concentrations between 5 and 8.3 g/L over the course of 12 days of culture.







Similar content being viewed by others
References
Walsh G (2018) Biopharmaceutical benchmarks 2018. Nat Biotech 36:1136–1145
Kelley B (2009) Industrialization of mAb production technologies. MAbs 1(5):443–552
Rader RA, Langer ES (2015) Biopharmaceutical manufacturing: historical and future trends in titers, yields, and efficiency in commercial-scale bioprocessing. BioProcess J 13(4):47–54
Xu J, Rehmann M, Xu M et al (2020) Development of an intensified fedbatch production platform with doubled titers using N-1 perfusion seed for cell culture manufacturing. Bioresour Bioprocess 7:17
Xu J, Xu X, Huang C et al (2020) Biomanufacturing evolution from conventional to intensified processes for productivity improvement: a case study. MAbs 12(1):669
Stepper L, Filser FA, Fischer S, Schaub J, Gorr I, Voges R (2020) Pre-stage perfusion and ultra-high seeding cell density in CHO fed-batch culture: a case study for process intensification guided by systems biotechnology. Bioprocess Biosyst Eng 43:1431–1443
Yang WC, Lu J, Kwiatkowski C et al (2014) Perfusion seed cultures improve biopharmaceutical fed-batch production capacity and product quality. Biotechnol Prog 30(3):616–625
Abu-Absi NR, Kenty BM, Cuellar ME, Borys MC, Sakhamuri S, Strachan DJ, Hausladen MC, Jian Li Z (2010) Real time monitoring of multiple parameters in mammalian cell culture bioreactor using an in-line Raman spectroscopy probe. Biotechnol Bioeng 108:1215–1221
Berry B, Moretto J, Matthews T, Smelko J, Wiltberger K (2015) Cross-scale predictive modeling of CHO cell culture growth and metabolites using Raman spectroscopy and multivariate analysis. Biotechnol Prog 31:566–577
Webster T, Hadley B, Hilliard W, Jaques C, Mason C (2018) Development of generic Raman models for GS-KO® CHO platform process. Biotechnol Prog 34:730–737
Rowland-Jones RC, Jaques C (2019) At-line Raman spectroscopy and design of experiments for robust monitoring and control of miniature bioreactor cultures. Biotechnol Prog 35:1–11
Mehdizadeh H, Lauri D, Karry KM, Moshgbar M, Procopio-Melion R, Drapeau D (2015) Generic Raman-based calibration models enabling real-time monitoring of cell culture bioreactors. Biotechnol Prog 31:1004–1013
Andre S, Lagresle S, Da Sliva A, Heimendinger P, Hannas Z, Calvosa E, Duponchel L (2017) Developing global regression models for metabolite concentration prediction regardless of cell line. Biotechnol Bioeng 114:2550–2559
Berry BN, Dobrowsky TM, Timson RC, Kshirsagar R, Ryll T, Wiltberger K (2016) Quick generation of Raman spectroscopy based in-process glucose control to influence biopharmaceutical protein product quality during mammalian cell culture. Biotechnol Prog 32:1–11
Rowland-Jones RC, van den Berg F, Racher AJ, Martin EB, Jaques C (2017) Comparison of spectroscopy technologies for improved monitoring of cell culture processes in miniature bioreactors. Biotechnol Prog 33:337–346
Matthews TE, Berry BN, Smelko J, Moretto J, Moore B, WiltbergerK, (2016) Closed loop control of lactate concentration in mammalian cell culture by raman spectroscopy leads to improved cell density, viability, and biopharmaceutical proteinproduction. Biotechnol Bioeng 113:2416–2424
Bhatia H, Mehdizadeh H, Drapeau D, Yoon S (2018) In-line monitoring of amino acids in mammalian cell cultures using Raman spectroscopy and multivariate chemometric models. Eng Life Sci 18:55–61
Webster TA, Hadley BC, Dickson M, Busa JK, Jaques C, Mason C (2021) Feedback control of two supplemental feeds during fed-batch culture on a platform process using inline Raman models for glucose and phenylalanine concentration. Bioprocess Biosyst Eng 44:127–140
Domján J, Pantea E, Gyürkés M, Gyurkes M, Madarasz L, Kozak D, Farkas A, Horvath B, Benko Z, Nagy ZK, Marosi G, Hirsch E (2022) Real-time amino acid and glucose monitoring system for the automatic control of nutrient feeding in CHO cell culture using Raman spectroscopy. Biotechnol J 17(5):1–14
Pereira S, Kildegaard HF, Andersen MR (2018) Impact of CHO metabolism on cell growth and protein production: an overview of toxic and inhibiting metabolites and nutrients. Biotechnol J 13:1–39
Lonza Biologics (2020) GS Gene Expression SystemTM brochure. https://www7.lonza.com/~/media/Files/CDM%2520Ferrell/The%2520Original%252GS%2520Gene%2520Expression%2520System%2520Brochure.ashx?la=en. Accessed 02 Apr 2020
Schneider M, Marison IW, von Stockar U (1996) The importance of ammonia in mammalian cell culture. J Biotechnol 46:161–185
Lao MS, Toth D (1997) Efects of ammonium and lactate on growth and metabolism of a recombinant chinese hamster ovary cell culture. Biotechnol Prog 13:688–691
Glacken MW, Fleischaker RJ, Sinskey AJ (1986) Reduction of waste product excretion via nutrient control: possible strategies for maximizing product and cell yields on serum in cultures of mammalian cells. Biotechnol Bioeng 28:1376
Chen P, Harcum SW (2006) Effects of elevated ammonium on glycosylation gene expression in CHO cells. Metab Eng 8:123–132
Carinhas N, Duarte TM, Barreiro LC, Carrondo MJT, Alves PM, Teixeira AP (2013) Metabolic signatures of GS-CHO cell clones associated with butyrate treatment and culture phase transition. Biotechnol Bioeng 110:3244–3257
Reinhart D, Damjanovic L, Kaisermayer C (2015) Kunert R (2015) Benchmarking of commercially available CHO cell culture media for antibody production. Appl Microbiol Biotechnol 99:4645–4657
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests directly or indirectly related to the work performed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Webster, T.A., Hadley, B.C., Dickson, M. et al. Automated Raman feed-back control of multiple supplemental feeds to enable an intensified high inoculation density fed-batch platform process. Bioprocess Biosyst Eng 46, 1457–1470 (2023). https://doi.org/10.1007/s00449-023-02912-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-023-02912-2