Abstract
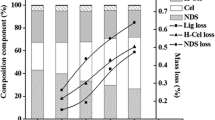
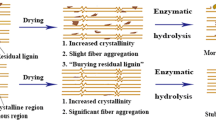
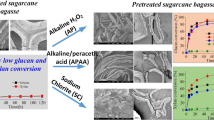
The underlying interplay between physicochemical property and enzymatic hydrolysis of cellulose still remains unclear. The impacts of matrix glycan composition of sugar beet pulp (SBP) on physical structure and saccharification efficiency were emphasized. The results showed that aqueous ammonia (AA) pretreatment could remove the non-cellulosic polysaccharides and destroy the linkage between the pectin and lignin. The cellulose supramolecule was changed significantly after AA pretreatment, in terms of the decline in hardness, gumminess, springiness, thickness and degree of polymerization. Furthermore, vascular cell was exposed and degraded. The highest reducing sugar yield of 355.06 mg/g was obtained from the pretreated SBP (80 °C) with enzyme loading of 30 U/g, which was 1.01 times higher than that of the untreated SBP. This research also supported the idea that recognizing and precisely removing the primary epitopes in cell walls might be an ideal strategy to accomplish the improved enzymatic hydrolysis through mild pretreatment.





Similar content being viewed by others
Data availability
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
References
Scavuzzo-Duggan T, Varoquaux N, Madera M, Vogel JP, Dahlberg J, Hutmacher R, Belcher M, Ortega J, Coleman-Derr D, Lemaux P, Purdom E, Scheller HV (2021) Cell wall compositions of Sorghum bicolor leaves and roots remain relatively constant under drought conditions. Front Plant Sci 12:747225. https://doi.org/10.3389/fpls.2021.747225
Haldar D, Purkait MK (2020) Lignocellulosic conversion into value-added products: a review. Process Biochem 89:110–133. https://doi.org/10.1016/j.biortech.2017.09.163
Himmel ME, Ding SY, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD (2007) Biomass recalcitrance: engineering plants and enzymes for biofuels production. Sci 315:804–807. https://doi.org/10.1126/science.1137016
Zhong R, Cui D, Ye ZH (2019) Secondary cell wall biosynthesis. New Phytol 221:1703–1723. https://doi.org/10.1111/nph.15537
Bhagia S, Ďurkovič J, Lagaňa R, Kardošová M, Kačík F, Cernescu A, Schäfer P, Yoo CG, Ragauskas AJ (2022) Nanoscale FTIR and mechanical mapping of plant cell walls for understanding biomass deconstruction. ACS Sustain Chem Eng 10:9. https://doi.org/10.1021/acssuschemeng.1c08163
Wang YF, Xu XY, Xue HT, Zhang DJ, Li GH (2021) Physical–chemical properties of cell wall interface significantly correlated to the complex recalcitrance of corn straw. Biotechnol Biofuels 14:1–10. https://doi.org/10.1186/s13068-021-02047-0
Petit J, Salentijn EMJ, Paulo MJ, Thouminot C, van Dinter BJ, Magagnini G, Gusovius HJ, Tang K, Amaducci S, Wang SL, Uhrlaub B, Müssig J, Trindade LM (2020) Genetic variability of morphological, flowering, and biomass quality traits in hemp (Cannabis sativa L.). Front Plant Sci 11:102. https://doi.org/10.3389/fpls.2020.00102
Puligundla P, Mok C (2021) Valorization of sugar beet pulp through biotechnological approaches: recent developments. Biotechnol Lett 43:1253–1263. https://doi.org/10.1007/s10529-021-03146-6
Li GH, He W, Yuan L (2017) Aqueous ammonia pretreatment of sugar beet pulp for enhanced enzymatic hydrolysis. Bioprocess Biosyst Eng 40:1603–1609. https://doi.org/10.1007/s00449-017-1816-9
Li GH, Sun YX, Guo WJ, Yuan L (2018) Comparison of various pretreatment strategies and their effect on chemistry and structure of sugar beet pulp. J Clean Prod 181:217–223. https://doi.org/10.1016/j.jclepro.2018.01.259
Du J, Anderson CT, Xiao C (2022) Dynamics of pectic homogalacturonan in cellular morphogenesis and adhesion, wall integrity sensing and plant development. Nat Plants 8:332–340. https://doi.org/10.1038/s41477-022-01120-2
da Costa RMF, Pattathil S, Avci U, Lee SJ, Hazen SP, Winters A, Hahn MG, Bosch M (2017) A cell wall reference profile for Miscanthus bioenergy crops highlights compositional and structural variations associated with development and organ origin. New Phytol 213:1710–1725. https://doi.org/10.1111/nph.14306
Liu Y, Xue H, Miao C, Li G (2022) Microstructural and histochemical variations of Korshinsk peashrub during aqueous ammonia pretreatment and its impacts on enzymatic digestibility of cellulose. J Environ Chem Eng 10:108613. https://doi.org/10.1016/j.jece.2022.108613
Hansen MAT, Hidayat BJ, Mogensen KK, Jeppesen MD, Jørgensen B, Johansen KS, Thygesen LG (2013) Enzyme affinity to cell types in wheat straw (Triticum aestivum L.) before and after hydrothermal pretreatment. Biotechnol. Biofuels 6:1–15. https://doi.org/10.1186/1754-6834-6-54
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Zhang W, Yi ZL, Huang JF, Li FC, Hao B, Li M, Hong SF, Lv YZ, Sun W, Ragauskas A, Hu F, Peng JH, Peng LC (2013) Three lignocellulose features that distinctively affect biomass enzymatic digestibility under NaOH and H2SO4 pretreatments in Miscanthus. Bioresour Technol 130:30–37. https://doi.org/10.1016/j.biortech.2012.12.029
Mo WX, Ke K, Shen X, Li B (2020) The influence of “thermal drying pretreatment” on enzymatic hydrolysis of cellulose and xylan in Poplar fibers with high lignin content. Carbohydr Polym 15:115400. https://doi.org/10.1016/j.carbpol.2019.115400
Dumitrache A, Tolbert A, Natzke J, Brown SD, Davison BH, Ragauskas AJ (2017) Cellulose and lignin colocalization at the plant cell wall surface limits microbial hydrolysis of Populus biomass. Green Chem 19:2275–2285. https://doi.org/10.1039/C7GC00346C
DeMartini JD, Pattathil S, Miller JS, Li H, Hahn MG, Wyman CE (2013) Investigating plant cell wall components that affect biomass recalcitrance in Poplar and switchgrass. Energy Environ Sci 6:898–909. https://doi.org/10.1039/c3ee23801f
Li GH, Zhang Y, Zhao C, Xue HT, Yuan L (2020) Chemical variation in cell wall of sugar beet pulp caused by aqueous ammonia pretreatment influence enzymatic digestibility of cellulose. Ind Crops Prod 155:112786. https://doi.org/10.1016/j.indcrop.2020.112786
Chandra R, Ewanick S, Hsieh C, Saddler JN (2008) The characterization of pretreated lignocellulosic substrates prior to enzymatic hydrolysis, part 1: a modified Simons’ staining technique. Biotechnol Prog 24:1178–1185. https://doi.org/10.1021/bp.33
Arantes V, Saddler JN (2011) Cellulose accessibility limits the effectiveness of minimum cellulase loading on the efficient hydrolysis of pretreated lignocellulosic substrates. Biotechnol Biofuels 4:1–17. https://doi.org/10.1186/1754-6834-4-3
Sun D, Alam A, Tu YY, Zhou SG, Wang YT, Xia T, Huang JF, Li Y, Zahoor WXY, Hao B, Peng LC (2017) Steam-exploded biomass saccharification is predominately affected by lignocellulose porosity and largely enhanced by Tween-80 in Miscanthus. Bioresour Technol 239:74–81. https://doi.org/10.1016/j.biortech.2017.04.114
Liu H, Chen XL, Ji GY, Yu HT, Gao CF, Han LJ (2019) Mechanochemical deconstruction of lignocellulosic cell wall polymers with ball-milling. Bioresour Technol 286:121364. https://doi.org/10.1016/j.biortech.2019.121364
Huang Y, Wei XY, Zhou SG, Liu MY, Tu YY, Li A, Chen P, Wang YT, Zhang XW, Tai HY, Peng LC, Xia T (2015) Steam explosion distinctively enhances biomass enzymatic saccharification of cotton stalks by largely reducing cellulose polymerization degree in G. barbadense and G. hirsutum. Bioresour Technol 181:224–230. https://doi.org/10.1016/j.biortech.2015.01.020
Raghavendra SN, Swamy SR, Rastogi NK, Raghavarao KSMS, Kumar S, Tharanathan RN (2006) Grinding characteristics and hydration properties of coconut residue: a source of dietary fiber. J Food Eng 72:281–286. https://doi.org/10.1016/j.jfoodeng.2004.12.008
Wang JX, Cheng QZ, McNeel J, Jacobson P (2010) Water retention value measurements of cellulosic materials using a centrifuge technique. Bioresources 5:1945–1954. https://doi.org/10.15376/biores.5.3.1945-1954
Chabbert B, Terryn C, Herbaut M, Vaidya A, Habrant A, Paës G, Donaldson L (2018) Fluorescence techniques can reveal cell wall organization and predict saccharification in pretreated wood biomass. Ind Crops Prod 123:84–92. https://doi.org/10.1016/j.indcrop.2018.06.058
Leroy A, Falourd X, Foucat L, Méchin V, Guillon F, Paës G (2021) Evaluating polymer interplay after hot water pretreatment to investigate maize stem internode recalcitrance. Biotechnol Biofuels 14:1–18. https://doi.org/10.1186/s13068-021-02015-8
Dinand E, Chanzy H, Vignon MR (1996) Parenchymal cell cellulose from sugar beet pulp: preparation and properties. Cellulose 3:183–188. https://doi.org/10.1007/BF02228800
Wang YJ, Qiao GR, Xu J, Jin KM, Fan MY, Ding YL, Wei Q, Zhuo RY (2022) Anatomical characteristics and variation mechanisms on the thick-walled and dwarfed culm of shidu bamboo (Phyllostachys nidularia f. farcta). Front Plant Sci 13:876658. https://doi.org/10.3389/fpls.2022.876658
Bhargava A, Mansfield SD, Hall HC, Douglas CJ, Ellis BE (2010) MYB75 functions in regulation of secondary cell wall formation in the Arabidopsis inflorescence stem. Plant Physiol 154:1428–1438. https://doi.org/10.1104/pp.110.162735
Gua H, An R, Bao J (2018) Pretreatment refining leads to constant particle size distribution of lignocellulose biomass in enzymatic hydrolysis. Chem Eng J 352:198–205. https://doi.org/10.1016/j.cej.2018.06.145
Ding SY, Liu YS, Zeng Y, Himmel ME, Baker JO, Bayer EA (2012) How does plant cell wall nanoscale architecture correlate with enzymatic digestibility? Science 338:1055–1060. https://doi.org/10.1126/science.1227491
Rosgaard L, Pedersen S, Langston J, Akerhielm D, Cherry JR, Meyer AS (2007) Evaluation of minimal Trichoderma reesei cellulase mixtures on differently pretreated barley straw substrates. Biotechnol Prog 23:1270–1276. https://doi.org/10.1021/bp070329p
Acknowledgements
This research received financial support from National Natural Science Foundation of China (no. 21868018 and no. 22068028).
Author information
Authors and Affiliations
Contributions
XH: investigation, data curation, formal analysis; RQ data curation, writing; LY: investigation; LY: editing; GL: conceiving, writing, funding acquisition. XH and RQ contributed equally to this work. All authors contributed to the manuscript have read and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xue, H., Qin, R., Liu, Y. et al. An aggregated understanding of the influence of aqueous ammonia pretreatment on the physical deconstruction of cell walls in sugar beet pulp. Bioprocess Biosyst Eng 46, 1427–1435 (2023). https://doi.org/10.1007/s00449-023-02908-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-023-02908-y