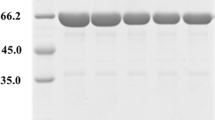
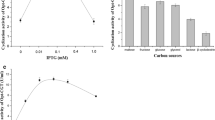
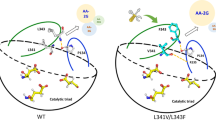
Abstract
2-O-α-D-glucopyranosyl-l-ascorbic acid (AA-2G) is a stable derivative of l-ascorbic acid (l-AA), which has been widely used in food and cosmetics industries. Sugar molecules, such as glucose and maltose produced by cyclodextrin glycosyltransferase (CGTase) during AA-2G synthesis may compete with l-AA as the acceptors, resulting in low AA-2G yield. Multiple sequence alignment combined with structural simulation analysis indicated that residues at positions 191 and 255 of CGTase may be responsible for the difference in substrate specificity. To investigate the effect of these two residues on the acceptor preference and the AA-2G yield, five single mutants Bs F191Y, Bs F255Y, Bc Y195F, Pm Y195F and Pm Y260F of three CGTases from Bacillus stearothermophilus NO2 (Bs), Bacillus circulans 251 (Bc) and Paenibacillus macerans (Pm) were designed for AA-2G synthesis. Under optimal conditions, the AA-2G yields of the mutants Bs F191Y and Bs F255Y AA-2G were 34.3% and 7.9% lower than that of Bs CGTase, respectively. The AA-2G yields of mutant Bc Y195F, Pm Y195F and Pm Y260F were 45.8%, 36.9% and 12.6% higher than those of wild-type CGTases, respectively. Kinetic studies revealed that the residues at positions 191 and 255 of the three CGTases were F, which decreased glucose and maltose specificity and increased l-AA specificity. This study not only proposes for the first time that the AA-2G yield can be improved by weakening the acceptor specificity of CGTase toward sugar byproducts, but also provides new insight on the modification of CGTase that catalyze the double-substrate transglycosylation reaction.





Similar content being viewed by others
Data availability
All relevant data are within the manuscript.
References
Yamamoto I, Muto N, Murakami K, Suga S, Yamaguchi H (1990) L-ascorbic acid alpha-glucoside formed by regioselective transglucosylation with rat intestinal and rice seed alpha-glucosidases: its improved stability and structure determination. Chem Pharm Bull 38:3020–3023. https://doi.org/10.1248/cpb.38.3020
Wenbin Z, Qicheng H, Ruijin Y, Wei Z, Xiao H (2021) 2-O-D-glucopyranosyl-L-ascorbic acid: properties, production, and potential application as a substitute for L-ascorbic acid. J Funct Foods 82:104481. https://doi.org/10.1016/j.jff.2021.104481
Song K, Sun J, Wang W, Hao J (2021) Heterologous expression of cyclodextrin glycosyltransferase my20 in Escherichia coli and its application in 2-O-alpha-d-glucopyranosyl-L-ascorbic acid production. Front Microbiol 12:664339. https://doi.org/10.3389/fmicb.2021.664339
Lee SB, Nam KC, Lee SJ, Lee JH, Inouye K, Park KH (2004) Antioxidative effects of glycosyl-ascorbic acids synthesized by maltogenic amylase to reduce lipid oxidation and volatiles—production in cooked chicken meat. Biosci Biotechnol Biochem 68:36–43. https://doi.org/10.1271/bbb.68.36
Zhou Y, Gan T, Jiang R, Chen H, Ma Z, Lu Y, Zhu L, Chen X (2022) Whole-cell catalytic synthesis of 2-O-alpha-glucopyranosyl-L-ascorbic acid by sucrose phosphorylase from Bifidobacterium breve via a batch-feeding strategy. Process Biochem 112:27–34. https://doi.org/10.1016/j.procbio.2021.11.023
Mukai K, Tsusaki K, Kubota M, Fukuda S, Miyake T (2014) Process for producing 2-O-alpha-D-glucopyranosyl-L-ascorbic acid
Tao X, Su L, Wu J (2019) Current studies on the enzymatic preparation 2-O-alpha-D-glucopyranosyl-L-ascorbic acid with cyclodextrin glycosyltransferase. Crit Rev Biotechnol 39:249–257. https://doi.org/10.1080/07388551.2018.1531823
Gudiminchi RK, Towns A, Varalwar S, Nidetzky B (2016) Enhanced synthesis of 2-O-alpha-D-glucopyranosyl-L-ascorbic acid from alpha-cyclodextrin by a highly disproportionating CGTase. Acs Catal 6:1606–1615. https://doi.org/10.1021/acscatal.5b02108
van der Veen BA, van Alebeek G-JWM, Uitdehaag JCM, Dijkstra BW, Dijkhuizen L (2000) The three transglycosylation reactions catalyzed by cyclodextrin glycosyltransferase from Bacillus circulans (strain 251) proceed via different kinetic mechanisms. Eur J Biochem 267:658–665. https://doi.org/10.1046/j.1432-1327.2000.01031.x
Leemhuis H, Dijkstra BW, Dijkhuizen L (2003) Thermoanaerobacterium thermosulfurigenes cyclodextrin glycosyltransferase—mechanism and kinetics of inhibition by acarbose and cyclodextrins. Eur J Biochem 270:155–162. https://doi.org/10.1046/j.1432-1033.2003.03376.x
Gaston JAR, Costa H, Rossi AL, Krymkiewicz N, Ferrarotti SA (2012) Maltooligosaccharides production catalysed by cyclodextrin glycosyltransferase from Bacillus circulans DF 9R in batch and continuous operation. Process Biochem 47:2562–2565. https://doi.org/10.1016/j.procbio.2012.08.008
Zhang Z, Li J, Liu L, Sun J, Hua Z, Du G, Chen J (2011) Enzymatic transformation of 2-O-alpha-D-glucopyranosyl-L-ascorbic acid by alpha-cyclodextrin glucanotransferase from recombinant Escherichia coli. Biotechnol Bioprocess Eng 16:107–113. https://doi.org/10.1007/s12257-010-0161-5
Xiong Y, Su L, Wang L, Wu J, Chen S (2015) Anchorage of cyclodextrin glycosyltransferase on outer membrane of Saccharomyces cerevisiae to produce 2-O-alpha-D-glucopyranosyl-L-ascorbic acid. Acta Microbiol Sin 55:1305–1313
Xu Q, Han R, Li J, Du G, Liu L, Chen J (2014) Improving maltodextrin specificity by site-saturation engineering of subsite +1 in cyclodextrin glycosyltransferase from Paenibacillus macerans. Chin J Biotechnol 30:98–108
Han R, Liu L, Shin H-d, Chen RR, Li J, Du G, Chen J (2013) Systems engineering of tyrosine 195, tyrosine 260, and glutamine 265 in cyclodextrin glycosyltransferase from Paenibacillus macerans To enhance maltodextrin specificity for 2-O-D-glucopyranosyl-L-ascorbic acid synthesis. Appl Environ Microbiol 79:672–677. https://doi.org/10.1128/aem.02883-12
Liu L, Xu Q, Han R, Shin H-d, Chen RR, Li J, Du G, Chen J (2013) Improving maltodextrin specificity for enzymatic synthesis of 2-O-D-glucopyranosyl-L-ascorbic acid by site-saturation engineering of subsite-3 in cyclodextrin glycosyltransferase from Paenibacillus macerans. J Biotechnol 166:198–205. https://doi.org/10.1016/j.jbiotec.2013.05.005
Han R, Liu L, Shin H-d, Chen RR, Li J, Du G, Chen J (2013) Iterative saturation mutagenesis of-6 subsite residues in cyclodextrin glycosyltransferase from Paenibacillus macerans to improve maltodextrin specificity for 2-O-D-glucopyranosyl-L-ascorbic acid synthesis. Appl Environ Microbiol 79:7562–7568. https://doi.org/10.1128/aem.02918-13
Tao X, Wang T, Su L, Wu J (2018) Enhanced 2-O-alpha-D-glucopyranosyl-L-ascorbic acid synthesis through iterative saturation mutagenesis of acceptor subsite residues in Bacillus stearothermophilus NO2 cyclodextrin glycosyltransferase. J Agric Food Chem 66:9052–9060. https://doi.org/10.1021/acs.jafc.8b03080
Chen S, Xiong Y, Su L, Wang L, Wu J (2017) Position 228 in Paenibacillus macerans cyclodextrin glycosyltransferase is critical for 2-O-D-glucopyranosyl-L-ascorbic acid synthesis. J Biotechnol 247:18–24. https://doi.org/10.1016/j.jbiotec.2017.02.011
Nakamura A, Haga K, Yamane K (1994) Four aromatic residues in the active center of cyclodextrin glucanotransferase from Alkalophilic Bacillus sp. 1011: effects of replacements on substrate binding and cyclization characteristics? Biochemistry 33:9929–9936. https://doi.org/10.1021/bi00199a015
Kanai R, Haga K, Akiba T, Yamane K, Harata K (2004) Role of Phe283 in enzymatic reaction of cyclodextrin glycosyltransferase from alkalophilic Bacillus sp. 1011: substrate binding and arrangement of the catalytic site. Protein Sci 13:457–465. https://doi.org/10.1110/ps.03408504
Yang Y, Wang L, Chen S, Wu J (2014) Optimization of beta-cyclodextrin production by recombinant beta-cyclodextrin glycosyltransferase. Biotechnol Bull 0:175–181
Li Z, Li B, Gu Z, Du G, Wu J, Chen J (2010) Extracellular expression and biochemical characterization of alpha-cyclodextrin glycosyltransferase from Paenibacillus macerans. Carbohydr Res 345:886–892. https://doi.org/10.1016/j.carres.2010.02.002
Tao X, Su L, Wang L, Chen X, Wu J (2020) Improved production of cyclodextrin glycosyltransferase from Bacillus stearothermophilus NO2 in Escherichia coli via directed evolution. Appl Microbiol Biotechnol 104:173–185. https://doi.org/10.1007/s00253-019-10249-8
Penninga D, Strokopytov B, Rozeboom HJ, Lawson CL, Dijkstra BW, Bergsma J, Dijkhuizen L (1995) Site-directed mutations in tyrosine 195 of cyclodextrin glycosyltransferase from Bacillus circulans strain 251 affect activity and product specificity. Biochemistry 34:3368–3376. https://doi.org/10.1021/bi00010a028
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (32001637, 31972032 and 31771916), the Natural Science Foundation of Jiangsu Province (BK20221536), the Agricultural independent innovation fund of Jiangsu Province (CX (21) 3039), and the Jiangnan University Basic Research Program (JUSRP122010).
Author information
Authors and Affiliations
Contributions
XT: conceptualization, methodology, data curation, visualization, writing-original draft; DK: methodology, visualization; HZ: formal analysis, writing—review and editing; LS: methodology, funding acquisition; SC: methodology, funding acquisition; DR: methodology; BW: methodology; JW: conceptualization, writing-review and editing; LW: conceptualization, supervision, writing-review and editing, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tao, X., Kong, D., Zhang, H. et al. Enhancing 2-O-α-D-glucopyranosyl-l-ascorbic acid synthesis by weakening the acceptor specificity of CGTase toward glucose and maltose. Bioprocess Biosyst Eng 46, 903–911 (2023). https://doi.org/10.1007/s00449-023-02875-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-023-02875-4