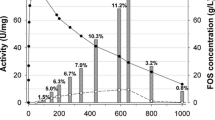
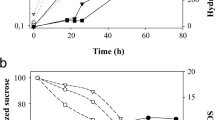
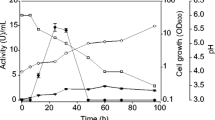
Abstract
Tyrosol and hydroxytyrosol, by-products of olive oil production, are valuable substrates for enzymatic transglycosylation that can provide products with pharmaceutical potential. Phenylethanoid fructosides are produced from sucrose and phenylethanoids by the catalytic action of β-fructofuranosidases. This work dealt with the potential of the most abundant β-fructofuranosidase, baker's yeast invertase, for this bioconversion. The effects of sucrose and phenylethanoid concentrations were investigated with a focus on the selectivity of phenylethanoid transfructosylation and fructoside yields. For this purpose, initial rate and progress curve experiments were carried out for the initial (hydroxy)tyrosol and sucrose concentrations of 0.072–0.3 M and 1–2 M, respectively. Reaction courses exhibited either a maximum or plateau of fructoside yield in the range of about 10–18%. The addition of deep eutectic solvents was applied in the concentration range from 5 to 70% (v/v) to investigate the possibility of shifting the reaction equilibrium towards fructoside synthesis.
Graphical abstract








Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ma GP, Zheng Q, Xu MB et al (2018) Rhodiola rosea L. improves learning and memory function: preclinical evidence and possible mechanisms. Front Pharmacol 9:1415. https://doi.org/10.3389/fphar.2018.01415
Karković Marković A, Torić J, Barbarić M, Jakobušić Brala C (2019) Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 24:1–39. https://doi.org/10.3390/molecules24102001
Emma MR, Augello G, di Stefano V et al (2021) Potential uses of olive oil secoiridoids for the prevention and treatment of cancer: a narrative review of preclinical studies. Int J Mol Sci 22:1–22. https://doi.org/10.3390/ijms22031234
Pedan V, Popp M, Rohn S et al (2019) Characterization of phenolic compounds and their contribution to sensory properties of olive oil. Molecules. https://doi.org/10.3390/molecules24112041
Horvathova E, Mastihubova M, Karnisova Potocka E et al (2019) Comparative study of relationship between structure of phenylethanoid glycopyranosides and their activities using cell-free assays and human cells cultured in vitro. Toxicol in Vitro. https://doi.org/10.1016/j.tiv.2019.104646
Kis P, Horváthová E, Gálová E et al (2021) Synthesis of tyrosol and hydroxytyrosol glycofuranosides and their biochemical and biological activities in cell-free and cellular assays. Molecules. https://doi.org/10.3390/molecules26247607
Akita H, Kawahara E, Kishida M, Kato K (2006) Synthesis of naturally occurring β-d-glucopyranoside based on enzymatic β-glycosidation. J Mol Catal B Enzym 40:8–15. https://doi.org/10.1016/j.molcatb.2006.01.031
Potocká E, Mastihubová M, Mastihuba V (2015) Enzymatic synthesis of tyrosol glycosides. J Mol Catal B Enzym 113:23–28. https://doi.org/10.1016/j.molcatb.2014.12.017
Wang F, Huang D, Ma Y et al (2019) Preparation of salidroside with n -butyl β -D-glucoside as the glycone donor via a two-step enzymatic synthesis catalyzed by immobilized β -glucosidase from bitter almonds. Biocatal Biotransform 37:246–260. https://doi.org/10.1080/10242422.2018.1549236
Bi Y, Wang Z, Mao Y et al (2012) Ionic liquid effects on the activity of β-glycosidase for the synthesis of salidroside in co-solvent systems. Chin J Catal 33:1161–1165. https://doi.org/10.1016/S1872-2067(11)60395-1
Panossian A, Wikman G, Sarris J (2010) Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 17:481–493. https://doi.org/10.1016/j.phymed.2010.02.002
Qi T, Gu G, Xu L et al (2017) Efficient synthesis of tyrosol galactosides by the β-galactosidase from Enterobacter cloacae B5. Appl Microbiol Biotechnol 101:4995–5003. https://doi.org/10.1007/s00253-017-8249-x
Hollá V, Hill R, Antošová M, Polakovič M (2021) Design of immobilized biocatalyst and optimal conditions for tyrosol β-galactoside production. Bioprocess Biosyst Eng 44:93–101. https://doi.org/10.1007/s00449-020-02425-2
Karnišová Potocká E, Mastihubová M, Mastihuba V (2019) Enzymatic synthesis of tyrosol and hydroxytyrosol β-d-fructofuranosides. Biocatal Biotransform 37:18–24. https://doi.org/10.1080/10242422.2017.1423060
Hollá V, Antošová M, Karkeszová K et al (2019) Screening of commercial enzymes for transfructosylation of tyrosol: effect of process conditions and reaction network. Biotechnol J. https://doi.org/10.1002/biot.201800571
Karnišová Potocká E, Mastihubová M, Mastihuba V (2021) Transrutinosylation of tyrosol by flower buds of Sophora japonica. Food Chem. https://doi.org/10.1016/j.foodchem.2020.127674
Toledo LET, García DM, Cruz EP, et al (2018) Fructosyltransferases and invertases: useful enzymes in the food and feed industries. In: Kuddus M (ed) Enzymes in food biotechnology: production, applications, and future prospects. Elsevier, pp 451–469
Antošová M, Polakovič M (2001) Fructosyltransferases: the enzymes catalyzing production. Chem Pap 55:350–358
Straathof AJJ, Kieboom APG, van Bekkum H (1986) Invertase-catalysed fructosyl transfer in concentrated solutions of sucrose. Carbohydr Res 146:154–159. https://doi.org/10.1016/0008-6215(86)85033-9
Vega-Paulino RJ, Zúniga-Hansen ME (2012) Potential application of commercial enzyme preparations for industrial production of short-chain fructooligosaccharides. J Mol Catal B Enzym 76:44–51. https://doi.org/10.1016/j.molcatb.2011.12.007
Marín-Navarro J, Talens-Perales D, Polaina J (2015) One-pot production of fructooligosaccharides by a Saccharomyces cerevisiae strain expressing an engineered invertase. Appl Microbiol Biotechnol 99:2549–2555. https://doi.org/10.1007/s00253-014-6312-4
Ganaie MA, Gupta US, Kango N (2013) Screening of biocatalysts for transformation of sucrose to fructooligosaccharides. J Mol Catal B Enzym 97:12–17. https://doi.org/10.1016/j.molcatb.2013.07.008
Lafraya Á, Sanz-Aparicio J, Polaina J, Marín-Navarro J (2011) Fructo-oligosaccharide synthesis by mutant versions of Saccharomyces cerevisiae invertase. Appl Environ Microbiol 77:6148–6157. https://doi.org/10.1128/AEM.05032-11
Breuer HJ, Bacon JSD (1957) Sucrose formation by Taka-diastase: action of the enzyme on methyl β-fructofuranoside and raffinose. Biochem J 66:462–468. https://doi.org/10.1042/bj0660462
Baseer A, Shall S (1971) The enzymic synthesis of fructosides using purified external β-fructosidase from Saccharomyces cerevisiae. Int J Biochem 2:503–506. https://doi.org/10.1016/0020-711X(71)90019-X
Straathof AJJ, Vrijenhoef JP, Sprangers EPAT et al (1988) Enzymic formation of β-D-fructofuranosides from sucrose: activity and selectivity of invertase in mixtures of water and alcohol. J Carbohydr Chem 7:223–238. https://doi.org/10.1080/07328308808058916
Selisko B, Ulbrich R, Schellenberger A, Müller U (1990) Invertase-catalyzed reactions in alcoholic solutions. Biotechnol Bioeng 35:1006–1010. https://doi.org/10.1002/bit.260351008
Rodríguez M, Gómez A, González F et al (1996) Selectivity of methyl-fructoside synthesis with β-fructofuranosidase. Appl Biochem Biotechnol 59:167–175. https://doi.org/10.1007/BF02787818
Abdul Manas NH, Md. Illias R, Mahadi NM (2018) Strategy in manipulating transglycosylation activity of glycosyl hydrolase for oligosaccharide production. Crit Rev Biotechnol 38:272–293. https://doi.org/10.1080/07388551.2017.1339664
Panić M, Cvjetko Bubalo M, Radojčić Redovniković I (2021) Designing a biocatalytic process involving deep eutectic solvents. J Chem Technol Biotechnol 96:14–30. https://doi.org/10.1002/jctb.6545
Kist JA, Zhao H, Mitchell-Koch KR, Baker GA (2021) The study and application of biomolecules in deep eutectic solvents. J Mater Chem B 9:536–566. https://doi.org/10.1039/d0tb01656j
Uhoraningoga A, Kinsella GK, Henehan GT, Ryan BJ (2021) β-glucosidase from Streptomyces griseus: ester hydrolysis and alkyl glucoside synthesis in the presence of Deep Eutectic Solvents. Curr Opin Green Sustain Chem. https://doi.org/10.1016/j.crgsc.2021.100129
Delavault A, Grüninger J, Kapp D et al (2022) Enzymatic synthesis of alkyl glucosides by β-glucosidases in a 2-in-1 deep eutectic solvent system. Chem Ing Tech 94:417–426. https://doi.org/10.1002/cite.202100150
Cheng QB, Zhang LW (2017) Highly efficient enzymatic preparation of daidzein in deep eutectic solvents. Molecules. https://doi.org/10.3390/molecules22010186
Hoppe J, Drozd R, Byzia E, Smiglak M (2019) Deep eutectic solvents based on choline cation—physicochemical properties and influence on enzymatic reaction with β-galactosidase. Int J Biol Macromol 136:296–304. https://doi.org/10.1016/j.ijbiomac.2019.06.027
Attya M, Benabdelkamel H, Perri E, Russo A, Sindona G (2010) Effects of conventional heating on the stability of major olive oil phenolic compounds by tandem mass spectrometry and isotope dilution assay. Molecules 15:8734–8746. https://doi.org/10.3390/molecules15128734
Zafra-Gómez A, Luzón-Toro B, Capel-Cuevas S, Carlos-Morales J (2011) Stability of hydroxytyrosol in aqueous solutions at different concentration, temperature and with different ionic content: a study using UPLC-MS. Food Nutr Sci 2:1114–1120. https://doi.org/10.4236/fns.2011.210149
Farine S, Versluis C, Bonnici PJ et al (2001) Application of high performance anion exchange chromatography to study invertase-catalysed hydrolysis of sucrose and formation of intermediate fructan products. Appl Microbiol Biotechnol 55:55–60. https://doi.org/10.1007/s002530000493
Bowski L, Saini R, Ryu DY, Vieth WR (1971) Kinetic modeling of the hydrolysis of sucrose by invertase. Biotechnol Bioeng 13:641–656. https://doi.org/10.1002/bit.260130505
Nelson JM, Schubert MP (1928) Water concentration and the rate of hydrolysis of sucrose by invertase. J Am Chem Soc 50:2189–2193. https://doi.org/10.1021/ja01395a017
Shearwin KE, Winzor DJ (1988) Substrate as a source of thermodynamic nonideality in enzyme kinetic studies: invertase-catalyzed hydrolysis of sucrose. Arch Biochem Biophys 260:532–539. https://doi.org/10.1016/0003-9861(88)90478-X
Antošová M, Illeová V, Vandáková M et al (2008) Chromatographic separation and kinetic properties of fructosyltransferase from Aureobasidium pullulans. J Biotechnol 135:58–63. https://doi.org/10.1016/j.jbiotec.2008.02.016
Polakovič M, Báleš V, Dluhý M, Štefuca V (1993) Bioprocess engineering optimization of a packed bed bioreactor with immobilized cells using experimental design. Bioprocess Eng 9:225–230. https://doi.org/10.1007/BF00369406
Valerio SG, Alves JS, Klein MP et al (2013) High operational stability of invertase from Saccharomyces cerevisiae immobilized on chitosan nanoparticles. Carbohydr Polym 92:462–468. https://doi.org/10.1016/j.carbpol.2012.09.001
Méndez-Líter JA, Tundidor I, Nieto-Domínguez M et al (2019) Transglycosylation products generated by Talaromyces amestolkiae GH3 β-glucosidases: effect of hydroxytyrosol, vanillin and its glucosides on breast cancer cells. Microb Cell Fact 18:1–12. https://doi.org/10.1186/s12934-019-1147-4
Gabriele F, Chiarini M, Germani R et al (2019) Effect of water addition on choline chloride/glycol deep eutectic solvents: characterization of their structural and physicochemical properties. J Mol Liq. https://doi.org/10.1016/j.molliq.2019.111301
Míguez N, Ramírez-Escudero M, Gimeno-Pérez M et al (2018) Fructosylation of hydroxytyrosol by the β-fructofuranosidase from Xanthophyllomyces dendrorhous: insights into the molecular basis of the enzyme specificity. ChemCatChem 10:4878–4887. https://doi.org/10.1002/cctc.201801171
Acknowledgements
This work was supported by grants from the Slovak Research and Development Agency (Grant No. APVV-18-0188) and the Slovak Grant Agency for Science (Grant No. VEGA 1/0515/22).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval
No ethical issues are related to the conducted research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karkeszová, K., Antošová, M., Potocká, E.K. et al. Medium engineering of phenylethanoid transfructosylation catalysed by yeast β-fructofuranosidase. Bioprocess Biosyst Eng 46, 237–249 (2023). https://doi.org/10.1007/s00449-022-02828-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-022-02828-3