Abstract
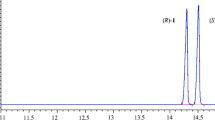
(R)-N-(2,6-dimethylphenyl) aminopropionic acid methyl ester ((R)-DMPM) is an important chiral intermediate of the fungicide N-(2,6-Dimethylphenyl)-N-(methoxyacetyl)-alanine methyl ester ((R)-Metalaxyl). In this study, (1) D3520 (macroporous acrylic anion resin), selected from the ten resins, was used to immobilize the esterase from Pseudochrobactrum asaccharolyticum WZZ003 (PAE07) for resoluting the (R,S)-DMPM to obtain (R)-DMPM. (2) Up to 20 g/L PAE07 could be immobilized onto D3520 with a high enzymatic activity of 32.4 U/g. Moreover, the Km and Vmax values of 19.1 mM and 2.8 mM/min for D3520-immobilized PAE07 indicated its high activity and stereoselectivity. (3) The optimal temperature and pH for the immobilized PAE07 were 40 ℃ and 8.0, and substrate concentration was up to 0.35 M. After 15 h reaction, the conversion rate from (R,S)-DMPM to (R)-DMPM was 48.0% and the e.e.p and E values were 99.5% and 1393.0, respectively. In scale-up resolution, 200 g/L substrate and 12.5 g immobilized esterase PAE07 condition, a conversion rate from substrate to product of 48.1% and a product e.e.p of 98% were obtained within 12 h, with the activity of immobilized PAE07 retained 80.2% after 5 cycles of reactions. These results indicated that the D3520-immobilized esterase PAE07 had great potential for enzymatic resolution of (R,S)-DMPM to prepare (R)-Metalaxyl.









Similar content being viewed by others
Data availability
The raw data used to support the findings of this study are available from the corresponding author upon request.
References
Monkiedje A, Ilori MO, Spiteller M (2002) Soil quality changes resulting from the application of the fungicides mefenoxam and metalaxyl to a sandy loam soil. Soil Biol Biochem 34:1939–1948
Martins MR, Santos C, Pereira P, Cruz-Morais J, Lima N (2017) Metalaxyl degradation by Mucorales strains Gongronella sp and Rhizopus oryzae. Molecules 22(12):2225
Celis R, Gamiz B, Adelino MA, Cornejo J, Hermosin MC (2015) Effect of formulation and repeated applications on the enantioselectivity of metalaxyl dissipation and leaching in soil. Pest Manag Sci 71:1572–1581
Yue H, Fang S, Zhang YZ, Ning Y, Yu WS, Kong FY, Qiu J (2016) Enantioselective effects of metalaxyl on soil enzyme activity. Chirality 28:771–777
Park OJ, Lee SH (2005) Stereoselective lipases from Burkholderia sp., cloning and - their application to preparation of methyl (R)-N-(2,6-dimethylphenyl)alaninate, a key intermediate for (R)-metalaxyl. J Biotechnol 120:174–182
Li K, Wang JH, He YJ, Abdulrazaq MA, Yan YJ (2018) Carbon nanotube-lipase hybrid nanoflowers with enhanced enzyme activity and enantioselectivity. J Biotechnol 281:87–98
Ye L, Liu X, Shen GH, Li SS, Luo QY, Wu HJ, Chen AJ, Liu XY, Li ML, Pu B, Qin W, Zhang ZQ (2019) Properties comparison between free and immobilized wheat esterase using glass fiber film. Int J Biol Macromol 125:87–91
Zhang YJ, Chen CS, Liu HT, Chen JL, Xia Y, Wu SJ (2019) Purification, identification and characterization of an esterase with high enantioselectivity to (S)-ethyl indoline-2-carboxylate. Biotechnol Lett 41:1223–1232
Bornscheuer UT (2002) Microbial carboxyl esterases: classification, properties and application in biocatalysis. Fems Microbiol Rev 26:73–81
Castro FF, Pinheiro ABP, Gerhardt ECM, Oliveira MAS, Barbosa-Tessmann IP (2018) Production, purification, and characterization of a novel serine-esterase from Aspergillus westerdijkiae. J Basic Microbiol 58:131–143
Chen PT, Liu CH, Chen YT, Hsu FY, Shaw JF (2020) Isolation, expression and characterization of the thermophilic recombinant esterase from geobacillus thermodenitrificans PS01. Appl Biochem Biotech 191:112–124
Park JM, Kang CH, Won SM, Oh KH, Yoon JH (2020) Characterization of a novel moderately thermophilic solvent-tolerant esterase isolated from a compost metagenome library. Front Microbiol 10:3069
Torres S, Martinez MA, Pandey A, Castro GR (2009) An organic-solvent-tolerant esterase from thermophilic Bacillus licheniformis S-86. Bioresource Technol 100:896–902
Lima GV, da Silva MR, de Sousa FT, de Lima LB, de Oliveira MdCF, de Lemos TLG, Zampieri D, dos Santos JCS, Rios NS, Gonçalves LRB, Molinari F, de Mattos MC (2017) Chemoenzymatic synthesis of (S)-Pindolol using lipases. Appl Catal A 546:7–14
Moreira KD, de Oliveira ALB, de Moura LS, de Sousa IG, Cavalcante ALG, Neto FS, Valerio RBR, Chaves AV, Fonseca TD, Cruz DMV, Lima GV, de Oliveira GP, de Souza MCM, Fechine PBA, de Mattos MC, da Fonseca AM, dos Santos JCS (2022) Taguchi design-assisted co-immobilization of lipase A and B from Candida antarctica onto chitosan: characterization, kinetic resolution application, and docking studies. Chem Eng Res Des 177:223–244
Lima PJM, da Silva RM, Neto C, Gomes ESNC, Souza J, Nunes YL, Sousa Dos Santos JC (2021) An overview on the conversion of glycerol to value-added industrial products via chemical and biochemical routes. Biotechnol Appl Biochem 1-25. https://doi.org/10.1002/bab.2098
Francisco Thálysson Tavares C, Aluisio Marques da F, Jeferson Yves Nunes Holanda A, José CSdS (2022) A stepwise docking and molecular dynamics approach for enzymatic biolubricant production using Lipase Eversa® Transform as a biocatalyst. Ind Crops Prod 187:115450
Fernandez-Lopez L, Bartolome-Cabrero R, Rodriguez MD, Dos Santos CS, Rueda N, Fernandez-Lafuente R (2015) Stabilizing effects of cations on lipases depend on the immobilization protocol. Rsc Adv 5:83868–83875
Rios NS, Neto DMA, Dos Santos JCS, Fechine PBA, Fernandez-Lafuente R, Goncalves LRB (2019) Comparison of the immobilization of lipase from Pseudomonas fluorescens on divinylsulfone or p-benzoquinone activated support. Int J Biol Macromol 134:936–945
Cheng F, Cheng FF, Zheng JY, Wu GZ, Zhang YJ, Wang Z (2018) A Novel esterase from Pseudochrobactrum asaccharolyticum WZZ003: enzymatic properties toward model substrate and catalytic performance in chiral fungicide intermediate synthesis. Process Biochem 69:92–98
Liu D-M, Dong C (2020) Recent advances in nano-carrier immobilized enzymes and their applications. Process Biochem 92:464–475
Boros Z, Weiser D, Markus M, Abahazioya E, Magyar A, Tomin A, Koczka B, Kovacs P, Poppe L (2013) Hydrophobic adsorption and covalent immobilization of Candida antarctica lipase B on mixed-function-grafted silica gel supports for continuous-flow biotransformations. Process Biochem 48:1039–1047
Nunes YL, de Menezes FL, de Sousa IG, Cavalcante ALG, Cavalcante FTT, da Silva MK, de Oliveira ALB, Mota GF, da Silva Souza JE, de Aguiar Falcão IR, Rocha TG, Valério RBR, Fechine PBA, de Souza MCM, dos Santos JCS (2021) Chemical and physical Chitosan modification for designing enzymatic industrial biocatalysts: how to choose the best strategy? Int J Biol Macromol 181:1124–1170
Zahirinejad S, Hemmati R, Homaei A, Dinari A, Hosseinkhani S, Mohammadi S, Vianello F (2021) Nano-organic supports for enzyme immobilization: scopes and perspectives. Colloids Surf, B 204:111774
Matsumoto T, Yamada R, Ogino H (2019) Chemical treatments for modification and immobilization to improve the solvent-stability of lipase. World J Microb Biot 35(12):193
Fernandez-Lafuente R, Armisen P, Sabuquillo P, Fernandez-Lorente G, Guisan JM (1998) Immobilization of lipases by selective adsorption on hydrophobic supports. Chem Phys Lipids 93:185–197
Goncalves D, Silva AG, Guidini CZ (2019) Lipases: sources, immobilization methods, and industrial applications. Appl Microbiol Biot 103:7399–7423
Zhang J, Cui H, Xue J, Wang W, Wang W, Le T, Chen L, Engelhardt UH, Jiang H (2021) Adsorption equilibrium and thermodynamics of tea theasinensins on HP20—a high-efficiency macroporous adsorption resin. Foods 10(12):2971
Liu D, Chen Z, Long J, Zhao Y, Du X (2018) Immobilization of penicillin acylase on macroporous adsorption resin CLX1180 carrier. Adv Polym Technol 37:753–760
Musdzalifah M, Fahrurrozi M, Sediawan WB, Susanti DY (2022) Separation of proanthocyanidin from red sorghum seed extract using macroporous resin. IOP Conference Series: Earth and Environmental science 963: 012043 (012045) 012043 (012045 pp.)
Shen N, Liu Y, Cui Y, Xin H (2022) Large-scale targetedly isolation of biflavonoids with high purity from industrial waste Ginkgo biloba exocarp using two-dimensional chromatography coupled with macroporous adsorption resin enrichment. Ind Crops Prod 175:114264
Zhang Y, Fan Y, Zhang W, Wu G, Wang J, Cheng F, Zheng J, Wang Z (2018) Bio-preparation of (R)-DMPM using whole cells of Pseudochrobactrum asaccharolyticum WZZ003 and its application on kilogram-scale synthesis of fungicide (R)-Metalaxyl. Biotechnol Prog 34:921–928
Cai HY, Li Y, Zhao MJ, Fu GW, Lai J, Feng FQ (2015) Immobilization, regiospecificity characterization and application of aspergillus oryzae lipase in the enzymatic synthesis of the structured lipid 1,3-Dioleoyl-2-Palmitoylglycerol. Plos One 10(7):e0133857
No DS, Zhao T, Lee J, Lee JS, Kim IH (2013) Synthesis of phytosteryl ester containing pinolenic acid in a solvent-free system using immobilized candida rugosa lipase. J Agr Food Chem 61:8934–8940
Rodrigues RC, Ortiz C, Berenguer-Murcia A, Torres R, Fernandez-Lafuente R (2013) Modifying enzyme activity and selectivity by immobilization. Chem Soc Rev 42:6290–6307
Lian WS, Li DM, Zhang L, Wang WF, Faiza M, Tan CP, Yang B, Lan DM, Wang YH (2018) Synthesis of conjugated linoleic acid-rich triacylglycerols by immobilized mutant lipase with excellent capability and recyclability. Enzyme Microb Tech 117:56–63
de Oliveira PC, Alves GM, de Castro HF (2000) Immobilisation studies and catalytic properties of microbial lipase onto styrene-divinylbenzene copolymer. Biochem Eng J 5:63–71
SreeHarsha N, Ghorpade RV, Alzahrani AM, Al-Dhubiab BE, Venugopala KN (2019) Immobilization studies of Candida Antarctica lipase B on gallic acid resin-grafted magnetic iron oxide nanoparticles. Int J Nanomed 14:3235–3244
Okobira T, Matsuo A, Matsumoto H, Tanaka T, Kai K, Minari C, Goto M, Kawakita H, Uezu K (2015) Enhancement of immobilized lipase activity by design of polymer brushes on a hollow fiber membrane. J Biosci Bioeng 120:257–262
Garcia-Galan C, Berenguer-Murcia A, Fernandez-Lafuente R, Rodrigues RC (2011) Potential of different enzyme immobilization strategies to improve enzyme performance. Adv Synth Catal 353:2885–2904
Srivastava P, Kaira G, Kapoor M (2017) Cross-linked enzyme aggregates (CLEAs) and magnetic nanocomposite grafted CLEAs of GH26 endo-β-1,4-mannanase: improved activity, stability and reusability. Int J Biol Macromol 105:1289–1299
Souza LTD, Moreno-Perez S, Lorente GF, Cipolatti EP, de Oliveira D, Resende RR, Pessela BC (2017) Immobilization of Moniliella spathulata R25L270 lipase on ionic, hydrophobic and covalent supports: functional properties and hydrolysis of sardine oil. Molecules 22(10):1508
Netto CGCM, da Silva DG, Toma SH, Andrade LH, Nakamura M, Araki K, Toma HE (2016) Bovine glutamate dehydrogenase immobilization on magnetic nanoparticles: conformational changes and catalysis (vol 6, pg 12977, 2016). Rsc Adv 6:51246–51246
Cipiciani A, Bellezza F, Fringuelli F, Silvestrini MG (2001) Influence of pH and temperature on the enantioselectivity of propan-2-ol-treated Candida rugosa lipase in the kinetic resolution of (+/-)-4-acetoxy- 2,2 -paracyclophane. Tetrahedron-Asymmetry 12:2277–2281
Sanchez A, Cruz J, Rueda N, dos Santos JCS, Torres R, Ortiz C, Villalonga R, Fernandez-Lafuente R (2016) Inactivation of immobilized trypsin under dissimilar conditions produces trypsin molecules with different structures. RSC Adv 6:27329–27334
Braham SA, Hussain F, Morellon-Sterling R, Kamal S, Kornecki JF, Barbosa O, Kati DE, Fernandez-Lafuente R (2019) Cooperativity of covalent attachment and ion exchange on alcalase immobilization using glutaraldehyde chemistry: enzyme stabilization and improved proteolytic activity. Biotechnol Prog 35:e2768
Chong SL, Cardoso V, Bras JLA, Gomes MZD, Fontes CMGA, Olsson L (2019) Immobilization of bacterial feruloyl esterase on mesoporous silica particles and enhancement of synthetic activity by hydrophobic-modified surface. Bioresour Technol 293:122009
Yemul O, Imae T (2005) Covalent-bonded immobilization of lipase on poly(phenylene sulfide) dendrimers and their hydrolysis ability. Biomacromol 6:2809–2814
Yao CY, Lin WJ, Yue KL, Ling XP, Jing KJ, Lu YH, Tang SK, Fan EG (2017) Biocatalytic synthesis of vitamin A palmitate using immobilized lipase produced by recombinant Pichia pastoris. Eng Life Sci 17:768–774
Zhong WC, Zhang MJ, Li XJ, Zhang YJ, Wang Z, Zheng JY (2020) Enantioselective resolution of (R, S)-2-Phenoxy-Propionic acid methyl ester by covalent immobilized lipase from Aspergillus oryzae. Appl Biochem Biotech 190:1049–1059
Reed MC, Lieb A, Nijhout HF (2010) The biological significance of substrate inhibition: a mechanism with diverse functions. BioEssays 32:422–429
Lu Y, Zhang Z, Zhang L, Lu Y (2016) Cloning and expression of an esterase gene from a new strain capable of enantioselective hydrolyzing methyl (R, S)-N-(2,6-dimethylphenyl) alaninate. Wei Sheng Wu Xue Bao 56:1335–1347
Park OJ, Lee SH, Park TY, Chung WG, Lee SW (2006) Development of a scalable process for a key intermediate of (R)-metalaxyl by enzymatic kinetic resolution. Org Process Res Dev 10:588–591
Torabizadeh H, Tavakoli M, Safari M (2014) Immobilization of thermostable α-amylase from Bacillus licheniformis by cross-linked enzyme aggregates method using calcium and sodium ions as additives. J Mol Catal B Enzym 108:13–20
Liu N, Fu M, Wang Y, Zhao QZ, Sun WZ, Zhao MM (2012) Immobilization of LecitaseA (R) ultra onto a novel polystyrene DA-201 resin: characterization and biochemical properties. Appl Biochem Biotech 168:1108–1120
Hou C, Zhu H, Wu DM, Li YJ, Hou K, Jiang Y, Li YF (2014) Immobilized lipase on macroporous polystyrene modified by PAMAM-dendrimer and their enzymatic hydrolysis. Process Biochem 49:244–249
Pinheiro MP, Rios NS, Fonseca TdS, Bezerra FdA, Rodriguez-Castellon E, Fernandez-Lafuente R, de Mattos MC, dos Santos JCS, Goncalves LRB (2018) Kinetic resolution of drug intermediates catalyzed by lipase B from Candida Antarctica immobilized on immobead-350. Biotechnol Prog 34:878–889
Galvao WS, Pinheiro BB, Golcalves LRB, de Mattos MC, Fonseca TS, Regis T, Zampieri D, dos Santos JCS, Costa LS, Correa MA, Bohn F, Fechine PBA (2018) Novel nanohybrid biocatalyst: application in the kinetic resolution of secondary alcohols. J Mater Sci 53:14121–14137
Manoel EA, dos Santos JCS, Freire DMG, Rueda N, Fernandez-Lafuente R (2015) Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzyme Microb Tech 71:53–57
Acknowledgements
The authors are thankful for Zhejiang Provincial Natural Science Foundation (LY18B020021) for financial support.
Author information
Authors and Affiliations
Contributions
Y-JZ: conceptualization, methodology, formal analysis, funding acquisition, investigation, writing-review and editing. L-TW: methodology, validation, formal analysis. M-PZ: methodology, validation, formal analysis, writing-original draft, visualization. CW: methodology, writing-review and editing, project administration. X-JY: conceptualization, writing-review and editing, project administration, supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, YJ., Wei, LT., Zhou, MP. et al. Enantioselective resolution of (R,S)-DMPM to prepare (R)-DMPM by an adsorbed-covalent crosslinked esterase PAE07 from Pseudochrobactrum asaccharolyticum WZZ003. Bioprocess Biosyst Eng 46, 171–181 (2023). https://doi.org/10.1007/s00449-022-02821-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-022-02821-w