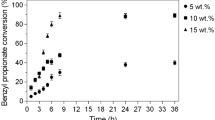
Abstract
The enzymatic production of isoamyl levulinate via esterification of isoamyl alcohol (IA) and levulinic acid (LA), a biomass-based platform chemical with attractive properties, in a solvent system has been performed in this study. For such a purpose, a low-cost liquid lipase (Eversa® Transform 2.0) immobilized by physical adsorption via hydrophobic interactions (mechanism of interfacial activation) on mesoporous poly(styrenene-divinylbenzene) (PSty-DVB) beads was used as heterogeneous biocatalyst. It was prepared at low ionic strength (5 mmol.L−1 buffer sodium acetate pH 5.0) and 25 ℃ using an initial protein loading of 40 mg.g−1 of support. Maximum protein loading of 31.2 ± 2.8 mg.g−1 of support and an immobilization yield of 83% was achieved. The influence of relevant factors (biocatalyst concentration and reaction temperature) on ester production was investigated using a central composite rotatable design (CCRD). Maximum acid conversion percentage of 65% was achieved after 12 h of reaction at 40 °C, 20% of mass of heterogeneous biocatalyst per mass of reaction mixture (20% m.m−1), and LA:IA molar ratio of 1:1.5 in a methyl isobutyl ketone (MIBK) medium. The biocatalyst retained around of 30% of its initial activity after five consecutive esterification batches under optimal experimental conditions. The proposed experimental procedure can be considered as an acceptable green process (EcoScale score of 66.5), in addition to the fact that a new strategy is proposed to sustainably produce a valuable industrial ester (isoamyl levulinate) from biomass-based materials using an immobilized and low-cost commercial lipase as catalyst.






Similar content being viewed by others
Data availability
The raw/processed data to support the findings of this study are included in the article.
References
Ramos MDN, Milessi TS, Candido RG et al (2022) Enzymatic catalysis as a tool in biofuels production in Brazil: current status and perspectives. Energy Sustain Dev 68:103–119. https://doi.org/10.1016/J.ESD.2022.03.007
Saravanan A, Senthil Kumar P, Jeevanantham S et al (2022) Recent advances and sustainable development of biofuels production from lignocellulosic biomass. Bioresour Technol 344:126203. https://doi.org/10.1016/J.BIORTECH.2021.126203
Badgujar KC, Badgujar VC, Bhanage BM (2022) Lipase as a green and sustainable material for production of levulinate compounds: state of the art. Mater Sci Energy Technol 5:232–242. https://doi.org/10.1016/J.MSET.2022.02.005
Khemthong P, Yimsukanan C, Narkkun T et al (2021) Advances in catalytic production of value-added biochemicals and biofuels via furfural platform derived lignocellulosic biomass. Biomass Bioenergy 148:106033. https://doi.org/10.1016/J.BIOMBIOE.2021.106033
Tian Y, Zhang F, Wang J et al (2021) A review on solid acid catalysis for sustainable production of levulinic acid and levulinate esters from biomass derivatives. Bioresour Technol 342:125977. https://doi.org/10.1016/J.BIORTECH.2021.125977
Luke Williams C, Westover TL, Emerson RM et al (2016) Sources of biomass feedstock variability and the potential impact on biofuels production. BioEnergy Res 9:1–14. https://doi.org/10.1007/s12155-015-9694-y
Schuster BG, Chinn MS (2013) Consolidated bioprocessing of lignocellulosic feedstocks for ethanol fuel production. BioEnergy Res 6:416–435. https://doi.org/10.1007/s12155-012-9278-z
Nahak BK, Preetam S, Sharma D et al (2022) Advancements in net-zero pertinency of lignocellulosic biomass for climate neutral energy production. Renew Sustain Energy Rev 161:112393. https://doi.org/10.1016/J.RSER.2022.112393
Aainaa N, Ramli S, Aishah N, Amin S (2020) Catalytic conversion of carbohydrate biomass in ionic liquids to 5-hydroxymethyl furfural and levulinic acid: a review. BioEnergy Res 13:693–736. https://doi.org/10.1007/s12155-020-10125-8
Sajid M, Farooq U, Bary G et al (2021) Sustainable production of levulinic acid and its derivatives for fuel additives and chemicals: progress, challenges, and prospects. Green Chem 23:9198–9238. https://doi.org/10.1039/d1gc02919c
Selifonov S (2013) Aducts of levulinic derivatives with epoxidized fatty acid esters and uses thereof. United States Patent US8436042B2
Rackemann DW, Bartley JP, Doherty WOS (2014) Methanesulfonic acid-catalyzed conversion of glucose and xylose mixtures to levulinic acid and furfural. Ind Crops Prod 52:46–57. https://doi.org/10.1016/J.INDCROP.2013.10.026
Quereshi S, Ahmad E, Pant KK, Dutta S (2017) Insights into the metal salt catalyzed ethyl levulinate synthesis from biorenewable feedstocks. Catal Today 291:187–194. https://doi.org/10.1016/J.CATTOD.2016.12.019
Song D, Sun Y, Zhang Q et al (2017) Fabrication of propylsulfonic acid functionalized SiO2 core/PMO shell structured PrSO3H-SiO2@Si(R)Si nanospheres for the effective conversion of d-fructose into ethyl levulinate. Appl Catal A Gen 546:36–46. https://doi.org/10.1016/J.APCATA.2017.08.004
Song D, An S, Sun Y, Guo Y (2016) Efficient conversion of levulinic acid or furfuryl alcohol into alkyl levulinates catalyzed by heteropoly acid and ZrO2 bifunctionalized organosilica nanotubes. J Catal 333:184–199. https://doi.org/10.1016/J.JCAT.2015.10.018
Tiwari MS, Dicks JS, Keogh J et al (2020) Direct conversion of furfuryl alcohol to butyl levulinate using tin exchanged tungstophosphoric acid catalysts. Mol Catal 488:110918. https://doi.org/10.1016/J.MCAT.2020.110918
Morawala DH, Dalai AK, Kalpana ·, et al (2020) Synthesis of n-butyl levulinate using mesoporous zeolite H-BEA catalysts with different catalytic characteristics. Catal Letters 3:1049–1060. https://doi.org/10.1007/s10562-019-03005-0
Di X, Zhang Y, Fu J et al (2019) Biocatalytic upgrading of levulinic acid to methyl levulinate in green solvents. Process Biochem 81:33–38. https://doi.org/10.1016/J.PROCBIO.2019.03.024
Salvi HM, Yadav GD (2019) Surface functionalization of SBA-15 for immobilization of lipase and its application in synthesis of alkyl levulinates: Optimization and kinetics. Biocatal Agric Biotechnol 18:101038. https://doi.org/10.1016/J.BCAB.2019.101038
Peixoto AF, Soliman MMA, Pinto TV et al (2021) Highly active organosulfonic aryl-silica nanoparticles as efficient catalysts for biomass derived biodiesel and fuel additives. Biomass Bioenergy 145:105936. https://doi.org/10.1016/J.BIOMBIOE.2020.105936
Zhao S, Wang Z, Chang C et al (2021) Enhanced production of levulinic acid/ester from furfural residue via pretreatment and two-stage alcoholysis. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-021-01307-1
Mthembu LD, Lokhat D, Deenadayalu N (2021) Esterification of levulinic acid to ethyl levulinate: optimization of process conditions using commercial levulinic acid and extension to the use of levulinic acid derived from depithed sugarcane bagasse. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-021-01632-5
Melchiorre M, Amendola R, Benessere V et al (2020) Solvent-free transesterification of methyl levulinate and esterification of levulinic acid catalyzed by a homogeneous iron(III) dimer complex. Mol Catal 483:110777. https://doi.org/10.1016/J.MCAT.2020.110777
Zhai S, Zhang L, Zhao X et al (2022) Enzymatic synthesis of a novel solid–liquid phase change energy storage material based on levulinic acid and 1,4-butanediol. Bioresour Bioprocess 9:12. https://doi.org/10.1186/S40643-022-00502-W
Akerman CO, Gaber Y, Ghani NA et al (2011) Clean synthesis of biolubricants for low temperature applications using heterogeneous catalysts. J Mol Catal B Enzym 72:263–269. https://doi.org/10.1016/j.molcatb.2011.06.014
Verger R (1997) ‘Interfacial activation’ of lipases: facts and artifacts. Trends Biotechnol 15:32–38. https://doi.org/10.1016/S0167-7799(96)10064-0
Schmid RD, Verger R (1998) Lipases: interfacial enzymes with attractive applications. Angew Chemie Int Ed 37:1608–1633. https://doi.org/10.1002/(SICI)1521-3773(19980703)37:12%3c1608::AID-ANIE1608%3e3.0.CO;2-V
Fernandez-Lafuente R (2010) Lipase from Thermomyces lanuginosus: uses and prospects as an industrial biocatalyst. J Mol Catal B Enzym 62:197–212
Adlercreutz P (2013) Immobilisation and application of lipases in organic media. Chem Soc Rev 42:6406–6436. https://doi.org/10.1039/c3cs35446f
Bilal M, Iqbal HMN (2019) Naturally-derived biopolymers: potential platforms for enzyme immobilization. Int J Biol Macromol 130:462–482. https://doi.org/10.1016/J.IJBIOMAC.2019.02.152
Rodrigues RC, Virgen-Ortíz JJ, dos Santos JCS et al (2019) Immobilization of lipases on hydrophobic supports: immobilization mechanism, advantages, problems, and solutions. Biotechnol Adv 37:746–770
Zhong L, Feng Y, Wang G et al (2020) Production and use of immobilized lipases in/on nanomaterials: a review from the waste to biodiesel production. Int J Biol Macromol 152:207–222. https://doi.org/10.1016/J.IJBIOMAC.2020.02.258
Monteiro RRC, Arana-Peña S, da Rocha TN et al (2021) Liquid lipase preparations designed for industrial production of biodiesel. Is it really an optimal solution? Renew Energy 164:1566–1587. https://doi.org/10.1016/J.RENENE.2020.10.071
Bolina ICA, Gomes RAB, Mendes AA (2021) Biolubricant production from several oleaginous feedstocks using lipases as catalysts: current scenario and future perspectives. BioEnergy Res 14:1039–1057. https://doi.org/10.1007/S12155-020-10242-4
Manoel EA, dos Santos JCS, Freire DMG et al (2015) Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzyme Microb Technol 71:53–57. https://doi.org/10.1016/J.ENZMICTEC.2015.02.001
Carvalho WCA, Luiz JHH, Fernandez-Lafuente R et al (2021) Eco-friendly production of trimethylolpropane triesters from refined and used soybean cooking oils using an immobilized low-cost lipase (Eversa® Transform 2.0) as heterogeneous catalyst. Biomass Bioenergy 155:106302. https://doi.org/10.1016/j.biombioe.2021.106302
Guedes Júnior JGE, Mattos FR, Sabi GJ et al (2022) Design of a sustainable process for enzymatic production of ethylene glycol diesters via hydroesterification of used soybean cooking oil. J Environ Chem Eng 10:107062. https://doi.org/10.1016/J.JECE.2021.107062
Sabi GJ, Gama RS, Fernandez-Lafuente R et al (2022) Decyl esters production from soybean-based oils catalyzed by lipase immobilized on differently functionalized rice husk silica and their characterization as potential biolubricants. Enzyme Microb Technol 157:110019. https://doi.org/10.1016/J.ENZMICTEC.2022.110019
Rodrigues RC, Berenguer-Murcia Á, Carballares D et al (2021) Stabilization of enzymes via immobilization: multipoint covalent attachment and other stabilization strategies. Biotechnol Adv 52:107821. https://doi.org/10.1016/J.BIOTECHADV.2021.107821
Bolivar JM, Woodley JM, Fernandez-Lafuente R (2022) Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem Soc Rev. https://doi.org/10.1039/d2cs00083k
Cerón AA, Vilas Boas RN, Biaggio FC, de Castro HF (2018) Synthesis of biolubricant by transesterification of palm kernel oil with simulated fusel oil: batch and continuous processes. Biomass Bioenergy 119:166–172. https://doi.org/10.1016/J.BIOMBIOE.2018.09.013
Vilas Bôas RN, de Lima R, Mendes AA et al (2021) Batch and continuous production of biolubricant from fusel oil and oleic acid: Lipase screening, reactor system development, and reaction optimization. Chem Eng Process: Process Intensif 168:108568. https://doi.org/10.1016/J.CEP.2021.108568
Dias ALB, Ubeyitogullari A, Hatami T et al (2021) Continuous production of isoamyl acetate from fusel oil under supercritical CO2: a mass transfer approach. Chem Eng Res Des 176:23–33. https://doi.org/10.1016/J.CHERD.2021.09.026
Wancura JHC, Fantinel AL, Ugalde GA et al (2021) Semi-continuous production of biodiesel on pilot scale via enzymatic hydroesterification of waste material: process and economics considerations. J Clean Prod 285:124838. https://doi.org/10.1016/J.JCLEPRO.2020.124838
Coppini M, Magro JD, Martello R et al (2019) Production of methyl esters by enzymatic hydroesterification of chicken fat industrial residue. Brazilian J Chem Eng 36:923–928. https://doi.org/10.1590/0104-6632.20190362s20180389
Yadav GD, Borkar IV (2008) Kinetic modeling of immobilized lipase catalysis in synthesis of n-butyl levulinate. Ind Eng Chem Res 47:3358–3363. https://doi.org/10.1021/IE800193F
Badgujar KC, Bhanage BM (2015) Thermo-chemical energy assessment for production of energy-rich fuel additive compounds by using levulinic acid and immobilized lipase. Fuel Process Technol 138:139–146. https://doi.org/10.1016/J.FUPROC.2015.05.015
Bhavsar KV (2018) Yadav GD (2018) n-Butyl levulinate synthesis using lipase catalysis: comparison of batch reactor versus continuous flow packed bed tubular microreactor. J Flow Chem 82(8):97–105. https://doi.org/10.1007/S41981-018-0014-5
Szelwicka A, Siewniak A, Kolanowska A et al (2021) PTFE-carbon nanotubes and lipase B from Candida Antarctica—long-lasting marriage for ultra-fast and fully selective synthesis of levulinate esters. Mater 14:1518. https://doi.org/10.3390/MA14061518
Kowalczykiewicz D, Szymańska K, Gillner D, Jarzębski AB (2021) Rotating bed reactor packed with heterofunctional structured silica-supported lipase. Developing an effective system for the organic solvent and aqueous phase reactions. Microporous Mesoporous Mater 312:110789. https://doi.org/10.1016/J.MICROMESO.2020.110789
Alves MD, Aracri FM, Cren ÉC, Mendes AA (2017) Isotherm, kinetic, mechanism and thermodynamic studies of adsorption of a microbial lipase on a mesoporous and hydrophobic resin. Chem Eng J 311:1–12. https://doi.org/10.1016/J.CEJ.2016.11.069
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Boudrant J, Woodley JM, Fernandez-Lafuente R (2020) Parameters necessary to define an immobilized enzyme preparation. Process Biochem 90:66–80. https://doi.org/10.1016/j.procbio.2019.11.026
Barbosa MS, Freire CCC, Brandão LMS et al (2021) Biolubricant production under zero-waste Moringa oleifera Lam biorefinery approach for boosting circular economy. Ind Crops Prod 167:113542. https://doi.org/10.1016/J.INDCROP.2021.113542
Van Aken K, Strekowski L, Patiny L, Strekowski L (2006) EcoScale a semi-quantitative tool to select an organic preparation based on economical and ecological parameters. Beilstein J Org Chem 2:1–7. https://doi.org/10.1186/1860-5397-2-3
Tischer W, Kasche V (1999) Immobilized enzymes: crystals or carriers? Trends Biotechnol 17:326–335. https://doi.org/10.1016/S0167-7799(99)01322-0
Sousa RR, Silva AS, Fernandez-Lafuente R, Ferreira-Leitão VS (2021) Solvent-free esterifications mediated by immobilized lipases: a review from thermodynamic and kinetic perspectives. Catal Sci Technol 11:5696–5711. https://doi.org/10.1039/D1CY00696G
Regan DL, Lilly MD, Dunnill P (1974) Influence of intraparticle diffuisional limitation on the observed kinetics of immobilized enzymes and on catalyst design. Biotechnol Bioeng 16:1081–1093. https://doi.org/10.1002/bit.260160808
Shen L, Chen Z (2007) Critical review of the impact of tortuosity on diffusion. Chem Eng Sci 62:3748–3755. https://doi.org/10.1016/j.ces.2007.03.041
Badgujar KC, Bhanage BM (2015) Immobilization of lipase on biocompatible co-polymer of polyvinyl alcohol and chitosan for synthesis of laurate compounds in supercritical carbon dioxide using response surface methodology. Process Biochem 50:1224–1236. https://doi.org/10.1016/J.PROCBIO.2015.04.019
Kumar A, Dhar K, Kanwar SS, Arora PK (2016) Lipase catalysis in organic solvents: advantages and applications. Biol Proced Online 18:2. https://doi.org/10.1186/S12575-016-0033-2
Mendoza-Ortiz PA, Gama RS, Gómez OC et al (2020) Sustainable enzymatic synthesis of a solketal ester—process optimization and evaluation of its antimicrobial activity. Catalysts 10:218. https://doi.org/10.3390/catal10020218
Jia B, Liu C, Qi X (2020) Selective production of ethyl levulinate from levulinic acid by lipase-immobilized mesoporous silica nanoflowers composite. Fuel Process Technol 210:106578. https://doi.org/10.1016/J.FUPROC.2020.106578
Páez BC, Medina AR, Rubio FC et al (2003) Modeling the effect of free water on enzyme activity in immobilized lipase-catalyzed reactions in organic solvents. Enzyme Microb Technol 33:845–853. https://doi.org/10.1016/S0141-0229(03)00219-9
Kim H, Choi N, Kim Y et al (2019) Immobilized lipase-catalyzed esterification for synthesis of trimethylolpropane triester as a biolubricant. Renew Energy 130:489–494. https://doi.org/10.1016/j.renene.2018.06.092
Virgen-Ortíz JJ, Tacias-Pascacio VG, Hirata DB et al (2017) Relevance of substrates and products on the desorption of lipases physically adsorbed on hydrophobic supports. Enzyme Microb Technol 96:30–35. https://doi.org/10.1016/J.ENZMICTEC.2016.09.010
Esfandmaz S, Chaibakhsh N, Moradi-Shoeili Z, Mohammadi A (2018) Eco-friendly synthesis of maleate ester: a comparison between solid acid and enzyme-catalyzed esterification. Sustain Chem Pharm 8:82–87. https://doi.org/10.1016/J.SCP.2018.03.003
Zhou L, He Y, Ma L et al (2018) Conversion of levulinic acid into alkyl levulinates: using lipase immobilized on meso-molding three-dimensional macroporous organosilica as catalyst. Bioresour Technol 247:568–575. https://doi.org/10.1016/J.BIORTECH.2017.08.134
Jiang Y, Liu H, Wang L et al (2019) Virus-like organosilica nanoparticles for lipase immobilization: characterization and biocatalytic applications. Biochem Eng J 144:125–134. https://doi.org/10.1016/J.BEJ.2019.01.022
Mesbah NM (2019) Covalent immobilization of a halophilic, alkalithermostable lipase LipR2 on Florisil® nanoparticles for production of alkyl levulinates. Arch Biochem Biophys 667:22–29. https://doi.org/10.1016/J.ABB.2019.04.004
Song M, Di X, Zhang Y et al (2021) The effect of enzyme loading, alcohol/acid ratio and temperature on the enzymatic esterification of levulinic acid with methanol for methyl levulinate production: a kinetic study. RSC Adv 11:15054–15059. https://doi.org/10.1039/D1RA01780B
Kuwahara Y, Kaburagi W, Nemoto K, Fujitani T (2014) Esterification of levulinic acid with ethanol over sulfated Si-doped ZrO2 solid acid catalyst: study of the structure–activity relationships. Appl Catal A Gen 476:186–196. https://doi.org/10.1016/J.APCATA.2014.02.032
Russo V, Rossano C, Salucci E et al (2020) Intraparticle diffusion model to determine the intrinsic kinetics of ethyl levulinate synthesis promoted by Amberlyst-15. Chem Eng Sci 228:115974. https://doi.org/10.1016/J.CES.2020.115974
Imyen T, Saenluang K, Dugkhuntod P, Wattanakit C (2021) Investigation of ZSM-12 nanocrystals evolution derived from aluminosilicate nanobeads for sustainable production of ethyl levulinate from levulinic acid esterification with ethanol. Microporous Mesoporous Mater 312:110768. https://doi.org/10.1016/J.MICROMESO.2020.110768
Methyl Isobutyl Ketone (MIBK) Price, Prices, Pricing | ChemAnalyst. https://www.chemanalyst.com/Pricing-data/methyl-isobutyl-ketone-68?msclkid=673b6d10c28e11ecadc996aa3ab489ea. Accessed 23 Apr 2022
Acknowledgements
The present study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) - Brazil - Finance Code 001. The authors also thank the financial support of Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG): Brazil (Process APQ-01691-21), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq): Brazil (Process 404929/2016-8). Marcus V. S. Cambraia thanks the CAPES Foundation for the student fellowship. Adriano A. Mendes thanks the CNPq Foundation for his research fellowship (PQ-2 CA EQ, Grant 310633/2020-6).
Author information
Authors and Affiliations
Contributions
MVS: visualization, investigation, data curation, and writing–original draft. MSB, CMFS, and AKFC: conceptualization, methodology, and writing–review & editing. AAM: conceptualization, supervision, funding acquisition, and writing–review & editing.
Corresponding author
Ethics declarations
Conflict interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cambraia, M.V.S., Barbosa, M.S., Soares, C.M.F. et al. Process optimization for enzymatic production of a valuable biomass-based ester from levulinic acid. Bioprocess Biosyst Eng 46, 53–67 (2023). https://doi.org/10.1007/s00449-022-02813-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-022-02813-w