Abstract
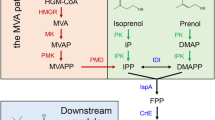
Dihydro-β-ionone is a characteristic aroma compound of Osmanthus fragrans and is widely applied in the flavor & fragrance industry. However, the main focus is on chemical synthesis due to the metabolic pathways of dihydro-β-ionone is still unclear. Here, we explored the one-pot synthesis system for dihydro-β-ionone production using carotenoid cleavage dioxygenase (CCD) and enoate reductase. After screening the CCD enzyme, PhCCD1 from the Petunia hybrid was identified as the suitable enzyme for the first step of dihydro-β-ionone synthesis due to the high enzyme activity for carotenoid. The PhCCD1 was expressed in Escherichia coli and further characterized. The optimal activity of PhCCD1 was observed at pH 6.8 and 45 °C. The enzyme was stable over the pH range of 6.0–8.0 and had good thermal stability below 40 °C. Then, we optimized the coupled reaction conditions for dihydro-β-ionone production by PhCCD1 and enoate reductase AaDBR1 from Artemisia annua. Furthermore, we introduced the NADPH regeneration system with a 1.5-fold enhancement for dihydro-β-ionone production. Collectively, approximately 13.34 mg/L dihydro-β-ionone was obtained by the one-pot biosystem with a corresponding molar conversion of 85.8%. For the first time, we successfully designed and constructed a new synthesis pathway for dihydro-β-ionone production in vitro. The coupled catalysis reported herein illustrates the feasibility of producing dihydro-β-ionone from carotenoids and guides further engineering in the food industry.









Similar content being viewed by others
References
Zheng X, Mi J, Deng X, Al-Babili S (2021) LC–MS-based profiling provides new insights into apocarotenoid biosynthesis and modifications in citrus fruits. J Agr Food Chem 69(6):1842–1851
Moreno JC, Mi J, Alagoz Y, Al-Babili S (2021) Plant apocarotenoids: from retrograde signaling to interspecific communication. Plant J 105(2):351–375
Wang J, Zhang N, Zhao M, Jing T, Jin J, Wu B, Wan X, Schwab W, Song C (2020) Carotenoid cleavage dioxygenase 4 catalyzes the formation of carotenoid-derived volatile beta-ionone during tea (Camellia sinensis) Withering. J Agric Food Chem 68(6):1684–1690
Ristic R, Bindon K, Francis LI, Herderich MJ, Iland PG (2010) Flavonoids and C13-norisoprenoids in Vitis vinifera L. cv. Shiraz: relationships between grape and wine composition, wine colour and wine sensory properties. Aust J Grape Wine R 16(3):369–388
Zhang X, Liao S, Cao F, Zhao L, Pei J, Tang F (2018) Cloning and characterization of enoate reductase with high β-ionone to dihydro-β-ionone bioconversion productivity. BMC Biotechnol 18(1):26
Lalko J, Lapczynski A, McGinty D, Bhatia S, Letizia CS, Api AM (2007) Fragrance material review on dihydro-β-ionone. Food Chem Toxicol 45(1):S225–S228
Odoux E, Grisoni M (2010) Fenaroli's handbook of flavor ingredients, sixth edition. Crc Press
Liu CH, Long LP, Hou XB (2011) Selective reduction of β-ionone during ultrasounic irridiation. J Cent S Unive (Sci Technol) 42(1):33–37
Knowles WS (2002) Asymmetric hydrogenations (Nobel Lecture). ChemInform 33(40):249–249
Schultes RE (1987) Common Fragrance and flavor materials: preparation, properties and uses. Econ Bot 41(4):493–493
Chapuis C, Jacoby D (2001) Catalysis in the preparation of fragrances and flavours. Appl Catal A Gen 221(1):93–117
Zhang CQ, Chen XX, Lindley ND, Too HP (2018) A “plug-n-play” modular metabolic system for the production of apocarotenoids. Biotechnol Bioeng 115(1):174–183
Cataldo VF, Lopez J, Carcamo M, Agosin E (2016) Chemical vs biotechnological synthesis of C-13-apocarotenoids: current methods, applications and perspectives. Appl Microbiol Biot 100(13):5703–5718
Ly MH, Hoang LC, Belin J-M, Waché Y (2008) Improved co-oxidation of β-carotene to β-ionone using xanthine oxidase-generated reactive oxygen species in a multiphasic system. Biotechnol J 3(2):220–225
Beekwilder J, van Rossum HM, Koopman F, Sonntag F, Buchhaupt M, Schrader J, Hall RD, Bosch D, Pronk JT, van Maris AJA, Daran JM (2014) Polycistronic expression of a beta-carotene biosynthetic pathway in Saccharomyces cerevisiae coupled to beta-ionone production. J Biotechnol 192:383–392
Ignea C, Raadam MH, Motawia MS, Makris AM, Vickers CE, Kampranis SC (2019) Orthogonal monoterpenoid biosynthesis in yeast constructed on an isomeric substrate. Nat Commun 10(1):3799
Wang X, Wu J, Chen J, Xiao L, Zhang Y, Wang F, Li X (2020) Efficient biosynthesis of R-(−)-Linalool through adjusting the expression strategy and increasing GPP supply in Escherichia coli. J Agr Food Chem 68(31):8381–8390
Zhou J, Wang C, Yang L, Choi E-S, Kim S-W (2015) Geranyl diphosphate synthase: an important regulation point in balancing a recombinant monoterpene pathway in Escherichia coli. Enzyme Microb Technol 68:50–55
Li R, Wang K, Wang D, Xu L, Shi Y, Dai Z, Zhang X (2021) Production of plant volatile terpenoids (rose oil) by yeast cell factories. Green Chem 23(14):5088–5096
Werner N, Ramirez-Sarmiento CA, Agosin E (2019) Protein engineering of carotenoid cleavage dioxygenases to optimize beta-ionone biosynthesis in yeast cell factories. Food Chem 299:125089
Lopez J, Bustos D, Camilo C, Arenas N, Saa PA, Agosin E (2020) Engineering Saccharomyces cerevisiae for the overproduction of beta-ionone and its precursor beta-carotene. Front Bioeng Biotechnol 8:578793
Lu Y, Yang Q, Lin Z, Yang X (2020) A modular pathway engineering strategy for the high-level production of beta-ionone in Yarrowia lipolytica. Microb Cell Fact 19(1):49
Gu N, Qiu C, Zhao L, Zhang L, Pei J (2020) Enhancing UDP-rhamnose supply for rhamnosylation of flavonoids in Escherichia coli by regulating the modular pathway and improving NADPH availability. J Agr Food Chem 68(35):9513–9523
Zhang J-D, Li A-T, Yu H-L, Imanaka T, Xu J-H (2011) Synthesis of optically pure S-sulfoxide by Escherichia coli transformant cells coexpressing the P450 monooxygenase and glucose dehydrogenase genes. J Ind Microbiol Biot 38(5):633–641
Li L, Wang X, Li X, Shi H, Wang F, Zhang Y, Li X (2019) Combinatorial engineering of mevalonate pathway and diterpenoid synthases in escherichia coli for cis-abienol production. J Agr Food Chem 67(23):6523–6531
Zang Y, Zha J, Wu X, Zheng Z, Ouyang J, Koffas MAG (2019) In vitro naringenin biosynthesis from p-coumaric acid using recombinant enzymes. J Agr Food Chem 67(49):13430–13436
Harrison PJ, Bugg TDH (2014) Enzymology of the carotenoid cleavage dioxygenases: Reaction mechanisms, inhibition and biochemical roles. Arch Biochem Biophys 544:105–111
Zhang XS, Pei JJ, Zhao LG, Tang F, Fang XY, Xie JC (2016) Overexpression and characterization of CCD4 from Osmanthus fragrans and beta-ionone biosynthesis from beta-carotene in vitro. J Mol Catal B-Enzym 134:105–114
Baldermann S, Kato M, Kurosawa M, Kurobayashi Y, Fujita A, Fleischmann P, Watanabe N (2010) Functional characterization of a carotenoid cleavage dioxygenase 1 and its relation to the carotenoid accumulation and volatile emission during the floral development of Osmanthus fragrans Lour. J Exp Bot 61(11):2967–2977
Huang FC, Molnar P, Schwab W (2009) Cloning and functional characterization of carotenoid cleavage dioxygenase 4 genes. J Exp Bot 60(11):3011–3022
Yahyaa M, Berim A, Isaacson T, Marzouk S, Bar E, Davidoyich-Rikanati R, Lewinsohn E, Ibdah M (2015) Isolation and functional characterization of carotenoid cleavage dioxygenase-1 from Laurus nobilis L. (Bay Laurel) Fruits. J Agr Food Chem 63(37):8275–8282
Czajka JJ, Nathenson JA, Benites VT, Baidoo EEK, Cheng QS, Wang YC, Tang YJJ (2018) Engineering the oleaginous yeast Yarrowia lipolytica to produce the aroma compound beta-ionone. Microb Cell Fact 17:136
Chen X, Shukal S, Zhang C (2019) Integrating enzyme and metabolic engineering tools for enhanced alpha-ionone production. J Agric Food Chem 67(49):13451–13459
Simkin AJ, Underwood BA, Auldridge M, Loucas HM, Shibuya K, Schmelz E, Clark DG, Klee HJ (2004) Circadian regulation of the PhCCD1 carotenoid cleavage dioxygenase controls emission of beta-ionone, a fragrance volatile of petunia flowers. Plant Physiol 136(3):3504–3514
Fleischmann P, Studer K, Winterhalter P (2002) Partial purification and kinetic characterization of a carotenoid cleavage enzyme from quince fruit (cydonia oblonga). J Agr Food Chem 50(6):1677–1680
Daruwalla A, Kiser PD (1865) 2020) Structural and mechanistic aspects of carotenoid cleavage dioxygenases (CCDs. Biochim Biophys Acta Mol Cell Biol Lipids 11:158590
Schilling M, Patett F, Schwab W, Schrader J (2007) Influence of solubility-enhancing fusion proteins and organic solvents on the in vitro biocatalytic performance of the carotenoid cleavage dioxygenase AtCCD1 in a micellar reaction system. Appl Microbiol Biot 75(4):829–836
Grubbe WS, Rasor BJ, Krüger A, Jewett MC, Karim AS (2020) Cell-free styrene biosynthesis at high titers. Metab Eng 61:89–95
Pei J, Sun Q, Gu N, Zhao L, Fang X, Tang F, Cao F (2020) Production of isoorientin and isovitexin from luteolin and apigenin using coupled catalysis of glycosyltransferase and sucrose synthase. Appl Biochem Biotech 190(2):601–615
Chaparro-Riggers JF, Rogers TA, Vazquez-Figueroa E, Polizzi KM, Bommarius AS (2007) Comparison of three enoate reductases and their potential use for biotransformations. Adv Synth Catal 349(8–9):1521–1531
Kosjek B, Fleitz FJ, Dormer PG, Kuethe JT, Devine PN (2008) Asymmetric bioreduction of α, β-unsaturated nitriles and ketones. Tetrahedron Asymmetry 19(12):1403–1406
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 32071711).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qi, Z., Tong, X., Zhang, X. et al. One-pot synthesis of dihydro-β-ionone from carotenoids using carotenoid cleavage dioxygenase and enoate reductase. Bioprocess Biosyst Eng 45, 891–900 (2022). https://doi.org/10.1007/s00449-022-02707-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-022-02707-x