Abstract
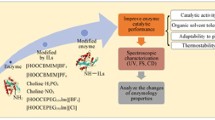
In this study, a series of proline ionic liquids with different lengths of hydrophobic alkyl on the side chain were used to modify the Candida Antarctic lipase B (CALB). The catalytic activity, thermal stability and tolerance to methanol and DMSO of the modified enzyme were all improved simultaneously. The optimum temperature changed from 55 to 60 ℃. The hydrophobicity and anion type of the modifier have important influence on the catalytic performance of CALB. CALB modified by [ProC12][H2PO4] has a better effect. Under the optimal conditions, its hydrolysis activity was 3.0 times than that of the native enzyme, the catalytic efficiency Kcat/Km improved 2.8 times in aqueous phase, and the tolerance to organic solvent with strong polarity (50% methanol 2 h) was increased by 6.8 times. Fluorescence spectra and circular dichroism (CD) spectroscopy showed that the introduction of ionic liquids changed the microenvironment near the fluorophores of the enzyme protein, the α-helix decreased and β-sheet increased in the secondary structure of the modified enzymes. The root mean square deviation (RMSD), residue root mean square fluctuation (RMSF), radius of gyration (Rg), and solution accessible surface area (SASA) of [ProC2][Br]-CALB, [ProC12][Br]-CALB and native CALB were obtained for comparison by molecular dynamics simulation. The results of dynamics simulation were in good agreement with enzymology experiment. The introduction of ionic liquids can keep CALB in a better active conformation, and proline ionic liquids with long hydrophobic chains can significantly improve the surface hydrophobicity and overall rigidity of CALB. This research offers a new idea for rapid screening of efficient modifiers and provision of enzymes with high stability and activity for industrial application.









Similar content being viewed by others
References
Sheldon RA, Pereira PC (2017) Biocatalysis engineering: the big picture. Chem Soc Rev 46:2678–2691
Choudhury P (2015) Industrial application of lipase: a review. BioPharm 1:41–47
Yang WK, Zhu LJ, Cui YC, Wang HG, Wang YW, Yuan L, Chen H (2016) Improvement of site-directed protein-polymer conjugates: high bioactivity and stability using a soft chain-transfer agent. ACS Appl Mater Interfaces 8:15967–15974
Diaz-Rodriguez A, Davis BG (2011) Chemical modification in the creation of novel biocatalysts. Curr Opin Chem Biol 15:211–219
Pagar AD, Patil MD, Flood DT, Yoo TH, Dawson PE, Yun H (2021) Recent advances in biocatalysis with chemical modification and expanded amino acid alphabet. Chem Rev 121:6173–6245
Basle E, Joubert N, Pucheault M (2010) Protein chemical modification on endogenous amino acids. Chem Biol 17:213–227
Nwagu TN, Aoyagi H, Okolo B, Moneke A, Yoshida S (2020) Citraconylation and maleylation on the catalytic and thermodynamic properties of raw starch saccharifying amylase from Aspergillus carbonarius. Heliyon 6:e04351
Jayawardena MB, Yee LH, Poljak A, Cavicchioli R, Kjelleberg SJ (2017) Siddiqui KS Enhancement of lipase stability and productivity through chemical modification and its application to latex-based polymer emulsions. Process Biochem 57:131–140
Bekhouche M, Blum LJ, Doumèche B (2011) Ionic liquid-inspired cations covalently bound to formate dehydrogenase improve its stability and activity in ionic liquids. ChemCatChem 3:875–882
Brogan APS, Bui-Le L, Hallett JP (2018) Non-aqueous homogenous biocatalytic conversion of polysaccharides in ionic liquids using chemically modified glucosidase. Nat Chem 10:859–865
Li XJ, Zhang C, Li S, Huang H, Hu Y (2015) Improving catalytic performance of Candida rugosa lipase by chemical modification with polyethylene glycol functional ionic liquids. Ind Eng Chem Res 54:8072–8079
Xu C, Yin XH, Zhang C, Chen HY, Huang H, Hu Y (2018) Improving catalytic performance of Burkholderiacepacia lipase by chemical modification with functional ionic liquids. Chem Res Chin Univ 34:279–284
Jia R, Hu Y, Liu L, Jiang L, Huang H (2013) Chemical modification for improving activity and stability of lipase B from Candida antarctica with imidazolium-functional ionic liquids. Org Biomol Chem 11:7192–7198
Jia R, Hu Y, Liu L, Jiang L, Zou B, Huang H (2013) Enhancing catalytic performance of porcine pancreatic lipase by covalent modification using functional ionic liquids. ACS Catal 3:1976–1983
Lv JP, Wei DQ, Wang YH, Xu Q (2016) Molecular dynamics method on thermostability of cold active Lipase 5. J At Mol Phys 33:128–134
Xie Y, An J, Yang GY, Wu G, Zhang Y, Cui L, Feng Y (2014) Enhanced enzyme kinetic stability by increasing rigidity within the active site. J Biol Chem 289:7994–8006
Evran S, Telefoncu A (2007) Modification of prcine pancreatic lipase with z-proline. Prep Biochem Biotechnol 35:191–201
Xu C, Suo HB, Xue Y, Qin J, Chen HY, Hu Y (2021) Experimental and theoretical evidence of enhanced catalytic performance of lipase B from Candida antarctica acquired by the chemical modification with amino acid ionic liquids. Mol Catal 501:111135
Noro J, Castro TG, Cavaco-Paulo A, Silva C (2020) Substrate hydrophobicity and enzyme modifiers play a major role in the activity of lipase from Thermomyces lanuginosus. Catal Sci Technol 10:5913–5924
Zhang L, Zhang HB, Luo HD, Zhou XH, Cheng GZ (2011) Novel chiral ionic liquid (CIL) assisted selectivity enhancement to (l)-proline catalyzed asymmetric aldol reactions. J Braz Chem Soc 9:1736–1741
Montalbetti CAGN, Falque V (2005) Amide bond formation and peptide coupling. Tetrahedron 61:10827–10852
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid—sciencedirect. Anal Biochem 150:76–85
Snydr SL, Sobocinski PZ (1975) An improved 2,4,6-trinitrobenzenesulfonic acid method for the determination of amines. Anal Biochem 1:284–288
Kordel M, Hofmann B, Schomburg D, Schmid RD (2019) Extracellular lipase of Pseudomonas sp. strain ATCC 21808: purification, characterization, crystallization, and preliminary X-ray diffraction data. J Bacteriol 173:4836–4841
Wu KJ, Odom RW (1998) MALDI MS is a powerful tool for determining molecular-weight distributions and structures of synthetic organic polymers. Anal Chem 70:456–461
Fenselau C, Demirev PA (2001) Characterization of intact microorganisms by MALDI mass spectrometry. Mass Spectrom Rev 20:157–171
Uppenberg J, Patkar S, Bergfors T, Jones TA (2011) Crystallization and preliminary X-ray studies of lipase B from Candida antarctica. J Mol Biol 235:790–792
Darden T, York D, Pedersen L (1993) Particle mesh Ewald: AnN⋅log(N) method for Ewald sums in large systems. J Chem Phys 98:10089–10092
Parrinello M, Rahman A (1981) Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys 52:7182–7190
Bussi G, Donadio D, Parrinello M (2007) Canonical sampling through velocity rescaling. J Chem Phys 126:014101
Ryckaert JP, Ciccotti G (1997) Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J Comput Phys 23:327–341
Yang Z (2009) Hofmeister effects: an explanation for the impact of ionic liquids on biocatalysis. J Biotechnol 144:12–22
Zhao H, Olubajo O, Song ZY, Sims AL, Person TE, Lawal RA, Holley LA (2006) Effect of kosmotropicity of ionic liquids on the enzyme stability in aqueous solutions. Bioorg Chem 34:15–25
Siddiqui KS, Cavicchioli R (2005) Improved thermal stability and activity in the cold-adapted lipase B from Candida antarctica following chemical modification with oxidized polysaccharides. Extremophiles 9:471–476
Forde J, Vakurovb A, Gibsonb TD, Millnerb P, Whelehana M, Marisona IW, O Fágáina C (2010) Chemical modificationand immobilisation of lipase B from Candida antarcticaonto mesoporous silicates. J Mol Catal B-Enzym 66:203–209
Goihberg E, Dym O, Tel-Or S, Levin I, Peretz M, Burstein Y (2007) A single proline substitution is critical for the thermostabilization of Clostridium beijerinckii alcohol dehydrogenase. Proteins 66:196–204
Kajiwara S, Komatsu K, Yamada R, Matsumoto T, Yasuda M, Ogino H (2019) Modification of lipase from Candida cylindracea with dextran using theborane-pyridine complex to improve organic solvent stability. J Biotechnol 296:1–6
Pogorevc M, Stecher H, Faber K (2002) A caveat for the use of log P values for the assessment of the biocompatibility of organic solvents. Biotech Lett 24:857–860
Veenstra TD, Johnson KL, Tomlinson AJ, Kumar R, Naylor S (1998) Correlation of fluorescence and circular dichroism spectroscopy with electrospray ionization mass spectrometry in the determination of tertiary conformational changes in calcium-binding proteins. Rapid Commun Mass Spectrom 12:613–619
Hassani L (2012) The effect of chemical modification with pyromellitic anhydride on structure, function, and thermal stability of horseradish peroxidase. Appl Biochem Biotechnol 167:489–497
Joondan N, Moosun SB, Caumul P, Sunassee SN, Venter GA, Jhaumeer-Laulloo S (2018) Effect of chain length on the interactions of sodium N-alkyl prolinates with bovine serum albumin: a spectroscopic investigation and molecular docking simulations. Colloid Polym Sci 296:367–378
Pan S, Liu X, Xie YD, Yi YY, Li C, Yan YJ, Liu Y (2010) Esterification activity and conformation studies of Burkholderia cepacia lipase in conventional organic solvents, ionic liquids and their co-solvent mixture media. Bioresour Technol 101:9822–9824
Miletic N, Loos K (2009) Over-Stabilization of chemically modified and cross-linked Candida antarctica lipase B using various epoxides and diepoxides. Aust J Chem 62:799–805
Wolynes P, Onuchic JN, Thirumalai D (1995) Navigating the folding routes. Science 267:1619–1920
Lobanov MY, Bogatyreva NS, Galzitskaya OV (2008) Radius of gyration as an indicator of protein structure compactness. Mol Biol 42:623–628
Longo MA, Combes D (1997) Influence of surface hydrophilic/hydrophobic balance on enzyme properties. J Biotechnol 58:21–32
Acknowledgements
This study was supported by the National Natural Science Foundation of China. (22178174, 21676143).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Xg., Xue, Y., Lu, Zp. et al. Chemical modification for improving catalytic performance of lipase B from Candida antarctica with hydrophobic proline ionic liquid. Bioprocess Biosyst Eng 45, 749–759 (2022). https://doi.org/10.1007/s00449-022-02696-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-022-02696-x