Abstract
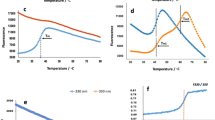
This study aimed to improve the catalytic activity of aspartate kinase (AK), the first key rate-limiting enzyme in the aspartic acid metabolism pathway, by site-directed saturation mutagenesis, and to weaken the synergistic feedback inhibition of metabolites and analyze its mechanism using molecular dynamics simulation (MD). The key residual sites around the inhibitor lysine (Lys) were selected to construct the mutant strains. The mutant A380M with significantly increased enzyme activity was obtained through enzyme activity screening. Kinetic analysis showed that the Vmax value increased to 15.73 U/mg, which was 4.8 times higher than that of wild-type AK (WT AK) (3.28 U/mg). The Kn value decreased to 0.61 mM, which was significantly lower than that of the wild type (4.77 mM), indicating that the substrate affinity increased. The enzyme properties analysis showed that the optimum temperature of the mutant A380M increased from 26 °C to 35 °C, the optimum pH remained unchanged. The stability was determined at optimum temperature (35 °C) and optimum pH 8.0, and it decreased from 4.8 h to 2.7 h. The feedback inhibition was weakened, showing a significant activation with the highest relative enzyme activity of 123.29% (Water was used instead of inhibitor as blank control group, and the highest enzyme activity was defined as 100%). Molecular dynamics simulations showed that the distance between ATP and Asp was shortened after mutation. The binding force and interaction between AK and ATP and substrate Asp were enhanced. The distance between catalytic residues D193 and S192 and substrate Asp was shortened.


modified by ESPript. Strictly conserved residues are highlighted by a red background. CpAK was made the alignment with CpAK (AK from Corynebacterium pekinensis), CgAK (AK from Corynebacterium glutamate), CaAK (AK from Clostridium acetobutylicum), ScAK (AK from Streptomyces coelicolor) and MtAK (AK from Mycobacterium TubercuLosis)






Similar content being viewed by others
References
Du HT, Zhao YQ, Wu FQ et al (2020) Engineering halomonas bluephagenesis for L-threonine production. Metab Eng 60:119–127
Leuchtenberger W, Klaus H, Karlheinz D (2005) Biotechnological production of amino acids and derivatives: current status and prospects. Appl Microbiol Biotechnol 69(1):1–8
Paris S, Viemon C, Curien G et al (2003) Mechanism of control of arabidopsis thaliana aspartate kinase-homoserine dehydrogenase by threonine. J Biol Chem 278(7):5361–5366
Petit C, Kim Y, Lee S et al (2018) Reduction of feedback inhibition in homoserine kinase (ThrB) of Corynebacterium glutamicum enhances l-threonine biosynthesis. ACS Omega 3(1):1178–1186
Becker J, Zelder O, Häfner S, Schröder H, Wittmann C (2011) From zero to hero–design-based systems metabolic engineering of Corynebacterium glutamicum for L-lysine production. Metab Eng 13(2):159–168
Becker J, Wittmann C (2012) Systems and synthetic metabolic engineering for amino acid production-the heartbeat of industrial strain development. Curr Opin Biotechnol 23:718–726
Dong XY, Peter J, Wang XY (2011) Metabolic engineering of Escherichia coli and Corynebacterium glutamicum for the production of L-threonine. Biotechnol Adv 29(1):11–23
Hermann T (2003) Industrial production of amino acids by coryneform bacteria. J Biotechnol 104(1–3):155–172
Park SD, Lee JY, Sim SY et al (2007) Characteristics of methionine production by an engineered Corynebacterium glutamicum strain. Metab Eng 9(4):327–336
Faehnle CR, Liu XY, Pavlovsky A, Viola RE (2006) The initial step in the archaeal aspartate biosynthetic pathway catalyzed by a monofunctional aspartokinase. Acta Crystallogr Sect F Struct Biol Cryst Commun 62:962–966
Chen Z, Rappert S, Sun JB, Zeng AP (2011) Integrating molecular dynamics and co-evolutionary analysis for reliable target prediction and deregulation of the allosteric inhibition of aspartokinase for amino acid production. J Biotechnol 154(4):248–254
Dong XY, Zhao Y, Zhao JX, Wang XY (2016) Characterization of aspartate kinase and homoserine dehydrogenase from Corynebacterium glutamicum IWJ001 and systematic investigation of L-isoleucine biosynthesis. J Ind Microbiol Biotechnol 43:873–885
Yoshioka Y, Kurei S, Machida Y (2001) Identification of a monofunctional aspartate kinase gene of Arabidopsis thaliana with spatially and temporally regulated expression. Genes Genet Syst 76(3):189–198
Robin AY, Cobessi D, Curien G et al (2010) A new mode of dimerization of allosteric enzymes with ACT domains revealed by the crystal structure of the aspartate kinase from Cyanobacteria. J Mol Biol 399(2):283–293
Galili G (1995) Regulation of lysine and threonine synthesis. Plant Cell 7:899–906
Richaud C, Mazat JP, Felenbok B, Patte JC (1974) The role of lysine and leucine binding on the catalytical and structural properties of aspartokinase III of Escherichia coli, K12. Eur J Biochem 48:147–156
Yoshida A, Tomita T, Kurihara T et al (2007) Structural insight into concerted inhibition of α2β2-type aspartate kinase from Corynebacterium glutamicum. J Mol Biology 368(2):521–536
Paris S, Wessel PM, Dumas R (2002) Overproduction, purification, and characterization of recombinant bifunctional threonine-sensitive aspartate kinase-homoserine de-hydrogenase from Arabidopsis thaliana, Protein Expr. Purif 24:105–110
Curien G, Laurencin M, Robert-Genthon M et al (2007) Allosteric monofunctional aspartate kinases from Arabidopsis. FEBS J 274(1):164–176
Kotaka M, Ren J, Lockyer M et al (2006) Structures of R- and T-state Escherichia coli Aspartokinase III: mechanisms of the allosteric transition and inhibition by lysine. J Biol Chem 281(42):31544–31552
Angeles TS, Viola RE (1990) The kinetic mechanisms of the bifunctional enzyme as-partokinase-homoserine dehydrogenase I from Escherichia coli. Arch Biochem Biophys 283:96–101
Veron M, Guillou Y, Cohen GN (1985) Isolation of the aspartokinase domain of bi-functional aspartokinase I-homoserine dehydrogenase I from E. Coli K12. FEBS Lett 181:381–384
Dumas R, Cobessi D, Robin AY et al (2012) The many faces of aspartate kinases. Arch Biochem Biophys 519(2):186–193
Kalinowski J, Cremer J, Bachmann B et al (1991) Genetic and biochemical analysis of the aspartokinase from Corynebacterium glutamicum. Mol Microbiol 5:1197–1204
Kandeel M, Kitade Y, Al-Taher A et al (2019) The structural basis of unique substrate recognition by Plasmodium thymidylate kinase: molecular dynamics simulation and inhibitory studies. PLoS ONE 14(2):1–16
Johnson P, Loganathan C, Iruthayaraj A et al (2018) S-allyl cysteine as potent anti-gout drug: insight into the xanthine oxidase inhibition and anti-inflammatory activity. Biochimie 154:1–9
Han CJ, Liu SM, Liu CL et al (2019) The mutant T379L of novel aspartokinase from Corynebacterium pekinense: a combined experimental and molecular dynamics simulation study. Process Biochem 83:77–85
Gao YN, Fang L, Min WH et al (2019) Enzymatic characterization and molecular mechanism of novel aspartokinase mutant M372I/T379W from Corynebacterium pekinense. RSC Adv 9:21344–21354
Labbé CM, Rey J, Lagorce D et al (2015) MTiOpenScreen: a web server for structure-based virtual screening. Nucleic Acids Res 43:W448–W454
Case DA, Iii TEC, Darden T et al (2005) The Amber biomolecular simulation programs. J Comput Chem 26(16):1668–1688
Solomon V, Teplitsky A, Shulami S et al (2007) Structure–specificity relationships of an intracellular xylanase from Geobacillus stearothermophilus. Acta Crystallogr A 63(8):845–859
Hess B, Bekker H, Berendsen HJC et al (1997) LINCS: a linear constraint solver for molecular simulations. J Comput Chem 18(12):1463–1472
Soares TA, Osman MA, Straatsma TP (2007) Molecular dynamics of organophosphorous hydrolases bound to the nerve agent soman. J Chem Theory Comput 3(4):1569–1579
Acknowledgements
This work is supported by Shandong Provincial Natural Science Foundation Youth Fund (Grant No. ZR2020QC217) and Jilin Provincial Education Department Science and Technology Project (Grant No. JJKH20210343KJ).
Author information
Authors and Affiliations
Contributions
All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fan, Z., Fang, L., Wu, L. et al. Improved catalytic activity of a novel aspartate kinase by site-directed saturation mutagenesis. Bioprocess Biosyst Eng 45, 541–551 (2022). https://doi.org/10.1007/s00449-021-02677-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02677-6