Abstract
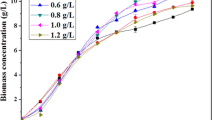
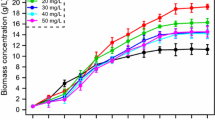
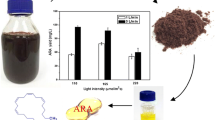
The red alga Porphyridium purpureum has been known to produce polyunsaturated fatty acids, especially arachidonic acid (ARA), under stressful conditions. However, there is no consistent conclusion about the response of ARA in this alga to nitrogen (N) stress. Also, no research has been done to clearly elucidate the underlying molecular mechanisms of N stress. In this work, P. purpureum CoE1 was cultivated under nitrogen limitation conditions and the putative Δ5-desaturase related gene FADSD5 was isolated. The results showed that the fatty acids in P. purpureum CoE1 were significantly higher in the N limited cultures (54.3 mg g−1) than in the N-replete cultures (45.3 mg g−1) at the 18th day (t-test, p < 0.001), which was attributed to the upregulated abundance of the putative Δ5-desaturase related protein, Δ5-Des. The study also indicated that the expression of the putative Δ5-desaturase related gene, FADSD5, increased with cell growth, demonstrating considerable potentials for ARA biosynthesis in P. purpureum CoE1. These results might guide the direction in illuminating the biosynthetic pathway of fatty acids with molecular evidence and enable genetic modifications of P. purpureum CoE1 for enhancing the ARA accumulation.





adapted from previous studies
Similar content being viewed by others
References
del Rio-Chanona EA, Wagner JL, Ali H, Fiorelli F, Zhang D, Hellgardt K (2019) Deep learning-based surrogate modeling and optimization for microalgal biofuel production and photobioreactor design. AIChE J 65:915–923
Kim SH, Sunwoo IY, Hong HJ, Awah CC, Jeong G-T, Kim S-K (2019) Lipid and unsaturated fatty acid productions from three microalgae using nitrate and light-emitting diodes with complementary LED wavelength in a two-phase culture system. Bioprocess Biosyst Eng 42:1517–1526
Chang J, Le K, Song X, Jiao K, Zeng X, Ling X, Shi T, Tang X, Sun Y, Lin L (2017) Scale-up cultivation enhanced arachidonic acid accumulation by red microalgae Porphyridium purpureum. Bioprocess Biosyst Eng 40:1763–1773
Guo D-S, Ji X-J, Ren L-J, Li G-L, Huang H (2017) Improving docosahexaenoic acid production by Schizochytrium sp. using a newly designed high-oxygen-supply bioreactor. AIChE J 63:4278–4286
Kralik Z, Kralik G, Grčević M, Hanžek D, Margeta P (2020) Microalgae Schizochytrium limacinum as an alternative to fish oil in enriching table eggs with n-3 polyunsaturated fatty acids. J Sci Food Agric 100:587–594
Cohen Z (1990) The production potential of eicosapentaenoic and arachidonic acids by the red alga Porphyridium cruentum. J Am Oil Chem Soc 67:916–920
Solovchenko AE, Khozin-Goldberg I, Didi-Cohen S, Cohen Z, Merzlyak MN (2008) Effects of light intensity and nitrogen starvation on growth, total fatty acids and arachidonic acid in the green microalga Parietochloris incisa. J Appl Phycol 20:245–251
Bigogno C, Khozingoldberg I, Boussiba S, Vonshak A, Cohen Z (2002) Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid. Phytochemistry 60:497–503
Jiao K, Chang J, Zeng X, Ng I, Xiao Z, Yong S, Xing T, Lu L (2017) 5-Aminolevulinic acid promotes arachidonic acid biosynthesis in the red microalga Porphyridium purpureum. Biotechnol Biofuels 10:168
Shanab SMM, Hafez RM, Fouad AS (2018) A review on algae and plants as potential source of arachidonic acid. J Advanc Res 11:3–13
Su G, Jiao K, Chang J, Li Z, Guo X, Sun Y, Zeng X, Lu Y, Lin L (2016) Enhancing total fatty acids and arachidonic acid production by the red microalgae Porphyridium purpureum. Bioresour Bioprocess 3:1–9
Su G, Jiao K, Li Z, Guo X, Chang J, Ndikubwimana T, Sun Y, Zeng X, Lu Y, Lin L (2016) Phosphate limitation promotes unsaturated fatty acids and arachidonic acid biosynthesis by microalgae Porphyridium purpureum. Bioprocess Biosyst Eng 39:1129–1136
Gong J, You F (2017) Optimal processing network design under uncertainty for producing fuels and value-added bioproducts from microalgae: Two-stage adaptive robust mixed integer fractional programming model and computationally efficient solution algorithm. AIChE J 63:582–600
Mondala AH, Hernandez R, French T, McFarland L, Santo Domingo JW, Meckes M, Ryu H, Iker B (2012) Enhanced lipid and biodiesel production from glucose-fed activated sludge: kinetics and microbial community analysis. AIChE J 58:1279–1290
Köst HP, Senser M, Wanner G (1984) Effect of nitrate and sulphate starvation on Porphyridium cruentum cells. Zeitschrift Für Pflanzenphysiologie 113:231–249
Breuer G, Lamers PP, Martens DE, Draaisma RB, Wijffels RH (2012) The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains. Biores Technol 124:217–226
Zhao LS, Li K, Wang QM, Song XY, Su HN, Xie BB, Zhang XY, Huang F, Chen XL, Zhou BC (2017) Nitrogen starvation impacts the photosynthetic performance of Porphyridium cruentumas revealed by chlorophyll a fluorescence. Sci Rep 7:8542
Asgharpour M, Rodgers B, Hestekin J (2015) Eicosapentaenoic acid from Porphyridium cruentum: increasing growth and productivity of microalgae for pharmaceutical products. Energies 8:10487–10503
Guihéneuf F, Stengel DB (2015) Towards the biorefinery concept: Interaction of light, temperature and nitrogen for optimizing the co-production of high-value compounds in Porphyridium purpureum. Algal Res 10:152–163
Hu H, Wang HF, Ma LL, Shen XF, Zeng RJ (2018) Effects of nitrogen and phosphorous stress on the formation of high value LC-PUFAs in Porphyridium cruentum. Appl Microbiol Biotechnol 102:5763–5773
Sato N, Moriyama T, Mori N, Toyoshima M (2017) Lipid metabolism and potentials of biofuel and high added-value oil production in red algae. World J Microbiol Biotechnol 33:74
Nichols BW, Appleby RS (1969) The distribution of arachidonic acid in algae. Phytochemistry 8:1907–1915
Bhattacharya D, Price DC, Chan CX, Qiu H, Rose N, Ball S, Weber AP, Arias MC, Henrissat B, Coutinho PM (2013) Genome of the red alga Porphyridium purpureum. Nat Commun 4:1941
Jones RF, Speer HL, Kury W (1963) Studies on the growth of the red alga Porphyridium cruentum. Physiol Plant 16:636–643
Han A, Dai M, Kao S, Gan J, Li Q, Wang L, Zhai W, Wang L (2012) Nutrient dynamics and biological consumption in a large continental shelf system under the influence of both a river plume and coastal upwelling. Limnol Oceanogr 57:486–502
Wang XW, Liang JR, Luo CS, Chen CP, Gao YH (2014) Biomass, total lipid production, and fatty acid composition of the marine diatom Chaetoceros muelleri in response to different CO2 levels. Biores Technol 161:124–130
Bligh ELG, Dyer WJA (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Shi X, Xin L, Ling L, Li M, Palenik B, Lin S (2017) Transcriptomic and microRNAomic profiling reveals multi-faceted mechanisms to cope with phosphate stress in a dinoflagellate. ISME J 11:2209–2218
Shi X, Li L, Lin S (2018) Circadian and irradiance effects on expression of antenna protein genes and pigment contents in dinoflagellate Prorocentrum donghaiense (Dinophycae). Harmful Algae 75:27–34
Shi X, Liu L, Li Y, Xiao Y, Ding G, Lin S, Chen J (2018) Isolation of an algicidal bacterium and its effects against the harmful-algal-bloom dinoflagellate Prorocentrum donghaiense (Dinophyceae). Harmful Algae 80:72–79
Shi X, Li L, Guo C, Lin X, Li M, Lin S (2015) Rhodopsin gene expression regulated by the light dark cycle, light spectrum and light intensity in the dinoflagellate Prorocentrum. Front Microbiol 6:555
Lin S, Zhang H, Zhuang Y, Tran B, Gill J (2010) Spliced leader-based metatranscriptomic analyses lead to recognition of hidden genomic features in dinoflagellates. Proc Natl Acad Sci USA 107:20033
Borcard D, Gillet F, Legendre P (2011) Numerical ecology with R. Springer, New York
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. Stat Comput 1406:133–199
Hochberg Y (1988) A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75:800–802
R Development Core Team (2018) R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Open access available at: http://cran.r-project.org
Alvensleben NV, Magnusson M, Heimann K (2016) Salinity tolerance of four freshwater microalgal species and the effects of salinity and nutrient limitation on biochemical profiles. J Appl Phycol 28:861–876
Khozin I, Adlerstein D, Bigongo C, Heimer YM, Cohen Z (1997) Elucidation of the biosynthesis of eicosapentaenoic acid in the microalga Porphyridium cruentum (II. studies with radiolabeled precursors). Plant Physiol 114:223–230
Wang DZ, Zhang H, Zhang Y, Zhang SF (2014) Marine dinoflagellate proteomics: current status and future perspectives. J Proteomics 105:121–132
McLean TI (2013) “Eco-omics”: a review of the application of genomics, transcriptomics, and proteomics for the study of the ecology of harmful algae. Microb Ecol 65:901–915
Raja K, Patrick M, Gao Y, Madu D, Yang Y, Tsoi LC (2017) A review of recent advancement in integrating omics data with literature mining towards biomedical discoveries. J Genet Genom 2017:6213474
Venegas-Calerón M, Sayanova O, Napier JA (2010) An alternative to fish oils: metabolic engineering of oil-seed crops to produce omega-3 long chain polyunsaturated fatty acids. Prog Lipid Res 49:108–119
Ruizlopez N, Usher S, Sayanova OV, Napier JA, Haslam RP (2015) Modifying the lipid content and composition of plant seeds: engineering the production of LC-PUFA. Appl Microbiol Biotechnol 99:143–154
Wallis JG, Browse J (1999) The Δ8-desaturase of Euglena gracilis: an alternate pathway for synthesis of 20-carbon polyunsaturated fatty acids. Arch Biochem Biophys 365:307–316
Acknowledgements
We are grateful for funding supported by the special fund for Fujian Ocean High-Tech Industry Development (No. FJHJF-L-2018-1), China and the Natural Science Foundation of Fujian Province of China (Grant No. 2019J06005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiao, K., Xiao, W., Shi, X. et al. Molecular mechanism of arachidonic acid biosynthesis in Porphyridium purpureum promoted by nitrogen limitation. Bioprocess Biosyst Eng 44, 1491–1499 (2021). https://doi.org/10.1007/s00449-021-02533-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02533-7