Abstract
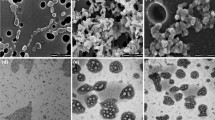
4-Allylpyrocatechol (APC), a major active compound of Piper betle, possesses strong antimicrobial activity. However, the water-insoluble property of APC limits its clinical and pharmaceutical use. To solve this problem, APC loaded polymeric micelles (PMAC) was fabricated using the thin-film hydration method. Nanoparticles of PMAC were characterized using a photon correlation spectrophotometer and transmission electron microscope (TEM). Antibiofilm activity of PMAC was investigated using crystal violet assay and confocal laser scanning microscopy (CLSM). Cytotoxic effects of PMAC on normal cells were investigated using MTT assay. The results demonstrate that a ratio of APC to the polymer plays an important role in the physicochemical characteristics of PMAC. The most suitable PMAC formulation having a small particle size (38.8 ± 1.4 nm), narrow size distribution (0.28 ± 0.10), a high negative zeta potential (− 16.43 ± 0.55 mV), and high entrapment efficiency (86.33 ± 14.27%) can be obtained from the ratio 1:4. The water solubility of this PMAC is significantly improved, approximately 1,000-fold higher than the unentrapped APC. TEM images demonstrate that PMAC is spherical in shape. The inhibitory effects of PMAC (1.5 mg APC/mL) against Streptococcus intermedius and Streptococcus mutans biofilms are significantly stronger than chlorhexidine (0.06 mg/mL). Images from CLSM demonstrate the destruction and thickness reduction of the pathogenic biofilms after contacting with PMAC. The MTT assay confirms that PMAC at this concentration is non-toxic to normal cells. These results obviously indicate that PMAC is a promising natural and harmless antimicrobial agent suitable for use in the oral cavity for inhibition of pathogenic bacterial biofilms.







Similar content being viewed by others
References
Takahashi N (2005) Microbial ecosystem in the oral cavity: metabolic diversity in an ecological niche and its relationship with oral diseases. Int Congr Ser 1248:103–112
Hurlbutt M, Novy B, Young D (2010) Dental caries : a pH-mediated diseases. Can Dent Hyg Assoc 25(1):9–15
Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C (2005) The global burden of oral diseases and risks to oral health. Bull World Health Organ 83(9):661–999
Takahashi N, Nyvad B (2008) Caries ecology revisited: microbial dynamics and the caries process. Caries Res 42(6):409–418
Brijendra S (2013) Gingivitis–a silent disease. IOSR J Dent Med Sci 6(5):30–33
Dowsett SA, Kowolik MJ, Archila LA, Eckert GJ, LeBlanc DJ (2002) Subgingival microbiota of indigenous Indians of Central America. J Clin Periodontol 29(2):159–167
Idrees MM, Azzeghaiby SN, Hammad MM, Kujan OB (2014) Prevalence and severity of plaque–induced gingivitis in a Saudi adult population. Saudi Med J 35(11):1373–1377
Kriebel K, Hieke C, Müller-Hilke B, Nakata M, Kreikemeyer B (2018) Oral biofilms from symbiotic to pathogenic interactions and associated disease–connection of periodontitis and rheumatic arthritis by peptidylarginine deiminase. Front Microbiol 9(53):1–14
Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, Galvin JL, Boches SK, Dewhirst FE, Griffen AL (2002) Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol 40(3):1001–1009
Hamada S, Slade HD (1980) Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev 44(2):331–384
Loesche WJ (1986) Role of Streptococcus mutans in human dental decay. Microbiol Rev 50(4):353–380
Jarvinen H, Tenovuo J, Huovinen P (1993) In vitro susceptibility of Streptococcus mutans to chlorhexidine and six other antimicrobial agents. Antimicrob Agents Chemother 37(5):1158–1159
Anantaworasakul P, Hamamoto H, Sekimizu K, Okonogi S (2017) In vitro antibacterial activity and in vivo therapeutic effect of Sesbania grandiflora in bacterial infected silkworms. Pharm Biol 55:1256–1262
Thanaseelungkoon N, Julsrigival J, Phannachet K, Chansakaow S (2018) Chemical compositions and biological activities of essential oils obtained from some Apiaceous and Lamiaceous plants collected in Thailand Asian Pac. J Trop Med 8:486–494
Okonogi S, Prakatthagomol W, Ampasavate C, Klayraung S (2011) Killing kinetics and bactericidal mechanism of action of Alpinia galanga on food borne bacteria. African J Microbiol Res 5(18):2847–2854
Pradhan D, Suri KA, Pradhan DK, Biswasroy P (2013) Golden heart of the nature : Piper betle L. J Pharmacogn Phytochem 1(6):147–167
Phumat P, Khongkhunthian S, Wanachantararak P, Okonogi S (2018) Effects of Piper betle fractionated extracts on inhibition of Streptococcus mutans and Streptococcus intermedius. Drug Discov Ther 12(3):133–141
Phumat P, Khongkhunthian S, Wanachantararak P, Okonogi S (2017) Potential of Piper betle extracts on inhibition of oral pathogens. Drug Discov Ther 11(6):307–315
Jesonbabu J, Spandana N, Lakshmi KA (2012) In vitro antimicrobial potentialities of chloroform extracts of ethanomedicinal plant against clinically isolated human pathogens. Int J Pharm Pharm Sci 4(3):624–662
Jesonbabu J, Spandana N, Lakshmi KA (2011) The potential activity of hydroxychavicol against pathogenic bacteria. J Bacteriol Parasitol 2(6):2–5
Okonogi S (2018) Nanoparticles of acitve components from plants. O.S.Printing House, Bangkok
Khonkarn R, Mankhetkorn S, Hennink WE, Okonogi S (2011) PEG–OCL micelles for quercetin solubilization and inhibition of cancer cell growth. Eur J Pharm Biopharm 79(2):268–275
Okonogi S (2012) Enhancement of anti–cholinesterase activity of Zingiber cassumunar essential oil using a microemulsion technique. Drug Discov Ther 6(5):249–255
Tima S, Anuchapreeda S, Ampasavate A, Berkland C, Okonogi S (2017) Stable curcumin–loaded polymeric micellar formulation for enhancing cellular uptake and cytotoxicity to FLT3 overexpressing EoL–1 leukemic cells. Eur J Pharm Biopharm 114:57–68
Anantaworasakul P, Okonogi S (2017) Encapsulation of Sesbania grandiflora extract in polymeric micelles to enhance its solubility, stability, and antibacterial activity. J Microencapsul 34(1):73–81
Sze A, Erickson D, Ren L, Li D (2003) Zeta–potential measurement using the Smoluchowski equation and the slope of the current–time relationship in electroosmotic flow. J Colloid Interface Sci 261:402–410
Okonogi S, Holzer W, Unger FM, Viernstein H, Mueller MKK (2016) Anti–inflammatory effects of compounds from Polygonum ordoratum. Nat Prod Commun 11(11):1651–1654
Okonogi S, Khonkarn R, Mankhetkorn S, Unger FM, Viernstein H (2013) Antioxidant activity and cytotoxicity of Cyrtosperma johnstonii extracts on drug sensitive and resistant leukemia and small cell lung carcinoma cells. Pharm Biol 51(3):329–338
Razak FA, Rahim ZHA (2003) The anti–adherence effect of Piper betle and Psidium guajava extracts on the adhesion of early settlers in dental plaque to saliva–coated glass surfaces. J Oral Sci 45(4):201–206
Nalina T, Rahim ZHA (2006) Effect of Piper betle L. leaf extract on the virulence activity of Streptococcus mutans–an in vitro study. Pak J Biol Sci 9(14):1470–1475
Acharya S, Sahoo SK (2011) PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Deliv Rev 63:170–183
Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, Khorasani S, Mozafari MR (2018) Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 10(2):1–17
Bhattacharjee S (2016) DLS and zeta potential–what they are and what they are not? J Control Release 235:337–351
Liu W, Guo R (2006) Interaction of flavonoid, quercetin with organized molecular assemblies of nonionic surfactant. Colloids Surf A Physicochem Eng Asp 274(1–3):192–199
Sharma S, Khan IA, Ali I, Ali F, Kumar M, Kumar A, Johri RK, Abdullah ST, Bani S, Pandey A, Suri KA, Gupta BD, Satti NK, Dutt P, Qazi GN (2009) Evaluation of the antimicrobial, antioxidant, and anti–inflammatory activities of hydroxychavicol for its potential use as an oral care agent. Antimicrob Agents Chemother 53(1):216–222
Gilbert JC, Hadgraft J, Bye A, Brookes LG (1986) Drug release from Pluronic F–127 gels. Int J Pharm 32(2–3):223–228
Sezgin Z, Yuksel N, Baykara T (2006) Preparation and characterization of polymeric micelles for solubilization of poorly soluble anticancer drugs. Eur J Pharm Biopharm 64(3):261–268
Butt AM, Amin MCIM, Katas H, Sarisuta N, Witoonsaridsilp W (2012) Benjakul R (2012) In vitro characterization of Pluronic F127 and D–α–tocopheryl polyethylene glycol 1000 succinate mixed micelles as nanocarriers for targeted anticancer-drug delivery. J Nanomater 916573:1–11
Patil PH, Wankhede PR, Mahajan HS, Zawar LR (2018) Aripiprazole–loaded polymeric micelles: fabrication, optimization and evaluation using response surface method. Recent Pat Drug Deliv Formul 12(1):53–64
Marsh PD (1994) Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res 8(2):263–271
Khonkarn R, Mankhetkorn S, Talelli M, Hennink WE, Okonogi S (2012) Cytostatic effect of xanthone-loaded mPEG-bp (HPMAm-Lac2) micelles towards doxorubicin sensitive and resistant cancer cells. Colloids Surfaces B Biointerfaces 94:266–273
Khonkarn R, Mankhetkorn S, Hennink WE, Okonogi S (2011) PEG-OCL micelles for quercetin solubilization and inhibition of cancer cell growth. Eur J Pharm Biopharm 79:268–275
Neu TR (1996) Significance of bacterial surface-active compounds in interaction of bacteria with interfaces. Microbiol Rev 60(1):151–166
Cerca N, Gomes F, Pereira S, Teixeira P, Oliveira R (2012) Confocal laser scanning microscopy analysis of S. epidermidis biofilms exposed to farnesol, vancomycin and rifampicin. BMC Res Notes. 5:1–7
Phumat P, Khongkhunthian S, Wanachantararak P, Okonogi S (2020) Comparative inhibitory effects of 4-allylpyrocatechol isolated from Piper betle on Streptococcus intermedius, Streptococcus mutans, and Candida albicans. Arch Oral Biol Elsevier 113:1–10
Harrison JJ, Ceri H, Yerly J, Stremick CA, Hu Y, Martinuzzi R et al (2006) The use of microscopy and three-dimensional visualization to evaluate the structure of microbial biofilms cultivated in the Calgary Biofilm Device. Biol Proced Online 8:194–215
Fulaz S, Vitale S, Quinn L, Casey E (2019) Nanoparticle–biofilm interactions: the role of the EPS matrix. Trends Microbiol 27(11):915–926
Tabatabaei FS, Moezizadeh M, Javand F (2015) Effects of extracts of Salvadora persica on proliferation and viability of human dental pulp stem cells. J Conserv Dent 18(4):315–320
Rodrigues S, Dionísio M, López CR, Grenha A (2012) Biocompatibility of chitosan carriers with application in drug delivery. J Funct Biomater 3:615–641
Rezvanian M, Amin MCIM, Ng SF (2016) Development and physicochemical characterization of alginate composite film loaded with simvastatin as a potential wound dressing. Carbohydr Polym 137:295–304
Tima S, Okonogi S, Ampasavate C, Berkland C, Anuchapreeda S (2019) FLT3-specific curcumin micelles enhance activity of curcumin on FLT3-ITD overexpressing MV4-11 leukemic cells. Drug Dev Ind Pharm 45:498–505
Acknowledgements
The authors are grateful to the Research Center of Pharmaceutical Nanotechnology, Chiang Mai University for their partial support. We also thank the Faculty of Pharmacy and Faculty of Dentistry, Chiang Mai University for facility and equipment supply.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Okonogi, S., Phumat, P. & Khongkhunthian, S. Enhancement of aqueous solubility and antibiofilm activity of 4-allylpyrocatechol by polymeric micelles. Bioprocess Biosyst Eng 44, 1289–1300 (2021). https://doi.org/10.1007/s00449-020-02501-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02501-7