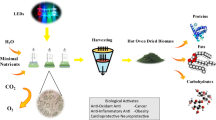
Abstract
The effect of light wavelengths on the physiological, biochemical and lutein content of the microalgal consortia Chlorella variabilis and Scenedesmus obliquus was evaluated using different light sources. Among different light treatments, cool-white fluorescent light produced the highest biomass of 673 mg L−1 with a specific growth rate of 0.75 day−1 followed by blue (500 mg L−1; 0.73 day−1). The chlorophyll content was enhanced under blue light (10.7 mg L−1) followed by cool fluorescent light (9.3 mg L−1), whereas the lutein productivity was enhanced under cool fluorescent light (7.22 mg g−1). Protein content of the microalgal consortia was enhanced under all light treatments with the highest protein accumulation under cool-white fluorescent light (~56% of dry mass) closely followed by amber light (52% of dry mass), whereas the carbohydrate content was higher under amber light (~35% of dry mass). The results revealed that the consortia could grow well on diluted dairy wastewater thereby reducing the cost of algal production when compared with the use of inorganic media and a two-phase culture process utilizing cool fluorescent and amber light could be employed for maximizing algal biomass and nutrient composition with enhanced lutein production. The study also emphasizes on the economic efficiency of LED lights in terms of biomass produced based on the modest electricity consumed and the importance of using amber light for cultivating microalgae for its nutrient content which has seldom been studied.







Similar content being viewed by others
References
Palacios YM, Vonshak A, Beardall J (2018) Photosynthetic and growth responses of Nannochloropsis oculata (Eustigmatophyceae) during batch cultures in relation to light intensity. Phycologia 57:492–502
Schulze PSC, Barreira LA, Pereira HGC, Perales JA, Varela JCS (2014) Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol 32:422–430
Ma R, Thomas-Hall SR, Chua ET, Netzel ME, Netzel G, Lu Y, Schenk PM (2018) LED power efficiency of biomass, fatty acid, and carotenoid production in Nannochloropsis microalgae. Biores Technol 252:118–126
Vadiveloo A, Moheimani NR, Cosgrove JJ, Bahri PA, Parlevliet D (2015) Effect of different light spectra on the growth and productivity of acclimated Nannochloropsis sp. (Eustigmatophyceae). Algal Res 8:121–127
Ueno Y, Aikawa S, Kondo A, Akimoto S (2019) Adaptation of light-harvesting functions of unicellular green algae to different light qualities. Photosynth Res 139:145–154
Krzemińska I, Pawlik-Skowrońska B, Trzcińska M, Tys J (2014) Influence of photoperiods on the growth rate and biomass productivity of green microalgae. Bioprocess Biosyst Eng 37:735–741
Figueroa FL, Aguilera J, Niell FX (1995) Red and blue light regulation of growth and photosynthetic metabolism in Porphyra umbilicalis (Bangiales, Rhodophyta). Eur J Phycol 30:11–18
Schulze PS, Pereira HG, Santos TF, Schueler L, Guerra R, Barreira LA, Perales JA, Varela JC (2016) Effect of light quality supplied by light emitting diodes (LEDs) on growth and biochemical profiles of Nannochloropsis oculata and Tetraselmis chuii. Algal Res 16:387–398
Ra CH, Sirisuk P, Jung JH, Jeong GT, Kim SK (2018) Effects of light-emitting diode (LED) with a mixture of wavelengths on the growth and lipid content of microalgae. Bioprocess Biosyst Eng 41:457–465
Wu H (2016) Effect of different light qualities on growth, pigment content, chlorophyll fluorescence, and antioxidant enzyme activity in the red alga Pyropia haitanensis (Bangiales, Rhodophyta). BioMed Res Int 20:1–8
Atta M, Idris A, Bukhari A, Wahidin S (2013) Intensity of blue LED light: a potential stimulus for biomass and lipid content in fresh water microalgae Chlorella vulgaris. Biores Technol 148:373–378
Zhong Y, Jin P, Cheng JJ (2018) A comprehensive comparable study of the physiological properties of four microalgal species under different light wavelength conditions. Planta 248:489–498
Teoa CL, Idrisa A, Wahidinb S, Laic LW (2014) Effect of different light wavelength on the growth of marine microalgae. J Technol 67:97–100
Gatamaneni BL, Orsat V, Lefsrud M (2018) Factors affecting growth of various microalgal species. Environ Eng Sci 35:1037–1048
Newby DT, Mathews TJ, Pate RC, Huesemann MH, Lane TW, Wahlen BD, Mandal S, Engler RK, Feris KP, Shurin JB (2016) Assessing the potential of polyculture to accelerate algal biofuel production. Algal Res 19:264–277
Ruangsomboon S (2012) Effect of light, nutrient, cultivation time and salinity on lipid production of newly isolated strain of the green microalga, Botryococcus braunii KMITL 2. Biores Technol 109:261–265
Cheng YS, Labavitch J, VanderGheynst JS (2015) Organic and inorganic nitrogen impact Chlorella variabilis productivity and host quality for viral production and cell lysis. Appl Biochem Biotechnol 176:467–479
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier R (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 111:1–61
Vidal G, Carvalho A, Mendez R, Lema JM (2000) Influence of the content in fats and proteins on the anaerobic biodegradability of dairy wastewaters. Biores Technol 74:231–239
Koreivienė J, Valčiukas R, Karosienė J, Baltrėnas P (2014) Testing of Chlorella/Scenedesmus microalgae consortia for remediation of wastewater, CO2 mitigation and algae biomass feasibility for lipid production. J Environ Eng Landsc Manag 22:105–114
Qin L, Sun Y, Shu Q, Feng P, Zhu L, Xu J, Yuan Z (2016) Microalgae consortia cultivation in dairy wastewater to improve the potential of nutrient removal and biodiesel feedstock production. Environ Sci Pollut Res 23:8379–8387
Parsons TR, Strickland JDH (1963) Discussion of spectrophotometric determination of marine plant pigments, with revised equation for ascertaining chlorophylls and carotenoids. J Mar Res 21:155–163
Porra R, Thompson W, Kriedemann P (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta Bioenerget 975:384–394
Macias-Sanchez MD, Mantell Serrano C, Rodriguez Rodriguez M, Martinez de la Ossa E, Lubian LM, Montero O (2008) Extraction of carotenoids and chlorophyll from microalgae with supercritical carbon dioxide and ethanol as cosolvent. J Sep Sci 31:1352–1362
Sartory DP, Grobbelaar JU (1984) Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 114:177–187
Hosikian A, Lim S, Halim R, Danquah MK (2010) Chlorophyll extraction from microalgae: a review on the process engineering aspects. Int J Chem Eng 20:1–11
Difusa A, Talukdar J, Kalita MC, Mohanty K, Goud VV (2015) Effect of light intensity and pH condition on the growth, biomass and lipid content of microalgae Scenedesmus species. Biofuels 6:37–44
Waghmare AG, Salve MK, LeBlanc JG, Arya SS (2016) Concentration and characterization of microalgae proteins from Chlorella pyrenoidosa. Bioresour Bioprocess 3:16
Slocombe SP, Ross M, Thomas N, McNeill S, Stanley MS (2013) A rapid and general method for measurement of protein in micro-algal biomass. Biores Technol 129:51–57
Folch J, Lees M, Sloane Stanley G (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Inbaraj BS, Chien JT, Chen BH (2006) Improved high performance liquid chromatographic method for determination of carotenoids in the microalga Chlorella pyrenoidosa. J Chromatogr A 1102:193–199
D'Este M, De Francisci D, Angelidaki I (2017) Novel protocol for lutein extraction from microalga Chlorella vulgaris. Biochem Eng J 127:175–179
French A, Macedo M, Poulsen J, Waterson T, Yu A (2008) Multivariate analysis of variance (MANOVA):1–8
Wang CY, Fu CC, Liu YC (2007) Effects of using light-emitting diodes on the cultivation of Spirulina platensis. Biochem Eng J 37:21–25
Gupta P, Sreelakshmi Y, Sharma R (2015) A rapid and sensitive method for determination of carotenoids in plant tissues by high performance liquid chromatography. Plant Methods 1:5
Amini Khoeyi Z, Seyfabadi J, Ramezanpour Z (2012) Effect of light intensity and photoperiod on biomass and fatty acid composition of the microalgae Chlorella vulgaris. Aquacult Int 20:41–49
Foyer (1984) Little light shed on photosynthesis. Photosynthesis 4:90135–90137
Kommareddy A, Anderson G (2003) Study of light as a parameter in the growth of algae in a photobioreactor (PBR). ASAE meeting Paper No. 034057: 1-23
Das P, Lei W, Aziz SS, Obbard JP (2011) Enhanced algae growth in both phototrophic and mixotrophic culture under blue light. Biores Technol 102:3883–3887
Ahluwalia A, Rai R, Kumar H (1980) Chromatic adaptation and photoreversal in blue-green alga Calothrix clavata West. J Biosci 2:63–68
Mercado JM, del Pilar S-S, Correa-Reyes G, Lubián L, Montero O, Figueroa FL (2004) Blue light effect on growth, light absorption characteristics and photosynthesis of five benthic diatom strains. Aquat Bot 78:265–277
Safafar H, Uldall Nørregaard P, Ljubic A, Møller P, Løvstad Holdt S, Jacobsen C (2016) Enhancement of protein and pigment content in two Chlorella species cultivated on industrial process water. J Mar Sci Eng 4:84
Marchetti J, Bougaran G, Jauffrais T, Lefebvre S, Rouxel C, Saint-Jean B, Lukomska E, Robert R, Cadoret JP (2013) Effects of blue light on the biochemical composition and photosynthetic activity of Isochrysis sp. (T-iso). J Appl Phycol 25:109–119
Sánchez JF, Fernández JM, Acién FG, Rueda A, Pérez-Parra J, Molina E (2008) Influence of culture conditions on the productivity and lutein content of the new strain Scenedesmus almeriensis. Process Biochem 43:398–405
Acknowledgements
The authors would like to thank the Natural Sciences and Engineering Research Council of Canada for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gatamaneni Loganathan, B., Orsat, V., Lefsrud, M. et al. A comprehensive study on the effect of light quality imparted by light-emitting diodes (LEDs) on the physiological and biochemical properties of the microalgal consortia of Chlorella variabilis and Scenedesmus obliquus cultivated in dairy wastewater. Bioprocess Biosyst Eng 43, 1445–1455 (2020). https://doi.org/10.1007/s00449-020-02338-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02338-0