Abstract
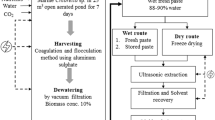
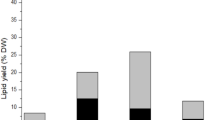
The objective of this work was to evaluate the effects of extrusion pretreatment on the efficiency of lipid extraction and the quality of lipid extracted from the microalga Nannochloropsis oceanica. Five processing parameters of extrusion pretreatment using a twin-screw extruder were studied: die configuration, distance from the internal surface of the die to the end of the screw (δ), temperature, screw rotation speed, and the water content of biomass. The results showed that die configuration, δ, and the water content of the biomass were vital factors affecting lipid extraction efficiency. After the pretreatment of extrusion, the extraction time was shortened from 10 h to 20 min, and the amount of solvent used was reduced to one-third of that without pretreatment. The content of polyunsaturated fatty acid (PUFA) in crude lipid increased by over 30 percent of those components without pretreatment, especially for eicosapentaenoic acid (EPA), which increased by 37.45%. In addition, the unsaponifiable content and acid value of the crude lipid extracted from the extruded microalga decreased significantly, which would facilitate further refining processes.





Similar content being viewed by others
References
Ma XN, Chen TP, Yang B, Liu J, Chen F (2016) Lipid production from nannochloropsis. Mar Drugs. https://doi.org/10.3390/md14040061
Huang Y, Zhang D, Xue S, Wang M, Cong W (2016) The potential of microalgae lipids for edible oil production. Appl Biochem Biotechnol 180(3):438–451. https://doi.org/10.1007/s12010-016-2108-6
Martins DA, Custodio L, Barreira L, Pereira H, Ben-Hamadou R, Varela J, Abu-Salah KM (2013) Alternative sources of n-3 long-chain polyunsaturated fatty acids in marine microalgae. Mar Drugs 11(7):2259–2281. https://doi.org/10.3390/md11072259
Domozych DS, Ciancia M, Fangel JU, Mikkelsen MD, Ulvskov P, Willats WG (2012) The cell walls of green algae: a journey through evolution and diversity. Front Plant Sci. https://doi.org/10.3389/fpls.2012.00082
Wang M, Cheng H, Chen S, Wen S, Wu X, Zhang D, Yuan Q, Cong W (2018) Microalgal cell disruption via extrusion for the production of intracellular valuables. Energy 142:339–345. https://doi.org/10.1016/j.energy.2017.10.061
Lee AK, Lewis DM, Ashman PJ (2012) Disruption of microalgal cells for the extraction of lipids for biofuels: processes and specific energy requirements. Biomass Bioenergy 46:89–101. https://doi.org/10.1016/j.biombioe.2012.06.034
Halim R, Danquah MK, Webley PA (2012) Extraction of oil from microalgae for biodiesel production: a review. Biotechnol Adv 30(3):709–732. https://doi.org/10.1016/j.biotechadv.2012.01.001
Middelberg AP (1995) Process-scale disruption of microorganisms. Biotechnol Adv 13(3):491–551. https://doi.org/10.1016/0734-9750(95)02007-P
Lee JY, Yoo C, Jun SY, Ahn CY, Oh HM (2010) Comparison of several methods for effective lipid extraction from microalgae. Bioresour Technol 101(Suppl 1):S75–77. https://doi.org/10.1016/j.biortech.2009.03.058
Halim R, Harun R, Danquah MK, Webley PA (2012) Microalgal cell disruption for biofuel development. Appl Energy 91(1):116–121. https://doi.org/10.1016/j.apenergy.2011.08.048
Topare NS, Raut SJ (2011) Extraction of oil from algae by solvent extraction and oil expeller method. Int J Chem Sci 9(4):1746–1750
Zhang X, Chen Y, Zhang R, Zhong Y, Luo Y, Xu S, Liu J, Xue J, Guo D (2016) Effects of extrusion treatment on physicochemical properties and in vitro digestion of pregelatinized high amylose maize flour. J Cereal Sci 68:108–115. https://doi.org/10.1016/j.jcs.2016.01.005
Ranjbar S, Basiri A, Elhamirad AH, Sharifi A, Chenarbon HA (2018) Effect of hydrocolloids on physicochemical, sensory and textural properties of reconstructed rice grain by extrusion cooking technology. J Food Meas Charact 12(3):1622–1632. https://doi.org/10.1007/s11694-018-9777-5
Moreno CR, Fernández PCR, Rodríguez EOC, Carrillo JM, Rochín SM (2018) Changes in nutritional properties and bioactive compounds in cereals during extrusion cooking. In: Extrusion of metals, polymers, and food products. IntechOpen, London. https://doi.org/10.5772/intechopen.68753
Jing Y, Chi YJ (2013) Effects of twin-screw extrusion on soluble dietary fibre and physicochemical properties of soybean residue. Food Chem 13(2–3):884–889. https://doi.org/10.1016/j.foodchem.2012.12.003
Yang Q (2015) Effect of extrusion treatment with different emulsifiers on the thermal stability and structure of corn starch. Czech J Food Sci 33(5):464–473. https://doi.org/10.17221/125/2015-CJFS
Evon P, Vandenbossche V, Pontalier PY, Rigal L (2009) Aqueous extraction of residual oil from sunflower press cake using a twin-screw extruder: feasibility study. Ind Crops Prod 29(2–3):455–465. https://doi.org/10.1016/j.indcrop.2008.09.001
Evon P, Amalia Kartika I, Cerny M, Rigal L (2013) Extraction of oil from jatropha seeds using a twin-screw extruder: feasibility study. Ind Crops Prod 47:33–42. https://doi.org/10.1016/j.indcrop.2013.02.034
Ding Q-B, Ainsworth P, Tucker G, Marson H (2005) The effect of extrusion conditions on the physicochemical properties and sensory characteristics of rice-based expanded snacks. J Food Eng 66(3):283–289. https://doi.org/10.1016/j.jfoodeng.2004.03.019
Yi J, Zhou L, Bi J, Liu X, Qinqin C, Wu X (2016) Influences of microwave pre-drying and explosion puffing drying induced cell wall polysaccharide modification on physicochemical properties, texture, microstructure and rehydration of pitaya fruit chips. LWT Food Sci Technol 70:271–279. https://doi.org/10.1016/j.lwt.2016.03.001
Zhang W-G, Jin G-M (2011) Microwave puffing-pretreated extraction of oil from Camellia oleifera seed and evaluation of its physicochemical characteristics. Int J Food Sci Technol. 46(12):2544–2549. https://doi.org/10.1111/j.1365-2621.2011.02779.x
Zhang W-G, Zhang D-C, Chen X-Y (2012) A novel process for extraction of tea oil from Camellia oleifera seed kernels by combination of microwave puffing and aqueous enzymatic oil extraction. Eur J Lipid Sci Technol 114(3):352–356. https://doi.org/10.1002/ejlt.201000304
Kawamoto J, Kurihara T, Yamamoto K, Nagayasu M, Tani Y, Mihara H, Hosokawa M, Baba T, Sato SB, Esaki N (2009) Eicosapentaenoic acid plays a beneficial role in membrane organization and cell division of a cold-adapted bacterium, Shewanella livingstonensis Ac10. J Bacteriol 191(2):632–640. https://doi.org/10.1128/JB.00881-08
Guschina IA, Harwood JL (2013) Algal lipids and their metabolism. Algae for biofuels and energy. Springer, London
Marventano S, Kolacz P, Castellano S, Galvano F, Buscemi S, Mistretta A, Grosso G (2015) A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: does the ratio really matter? Int J Food Sci Nutr 66(6):611–622. https://doi.org/10.3109/09637486.2015.1077790
Farhoosh R, Einafshar S, Sharayei P (2009) The effect of commercial refining steps on the rancidity measures of soybean and canola oils. Food Chem 115(3):933–938. https://doi.org/10.1016/j.foodchem.2009.01.035
Acknowledgements
This study was supported by the National Key R&D Program of China (2018YFD0401105).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there are no conflicts of interest in publishing this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Q., Zhou, Z., Zhang, D. et al. Lipid extraction from Nannochloropsis oceanica biomass after extrusion pretreatment with twin-screw extruder: optimization of processing parameters and comparison of lipid quality. Bioprocess Biosyst Eng 43, 655–662 (2020). https://doi.org/10.1007/s00449-019-02263-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-019-02263-x