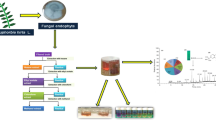
Abstract
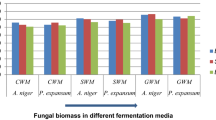
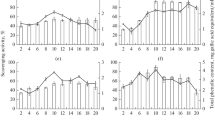
Antioxidants are able of inhibiting free radicals and play an important role in human diet, protection of foods, anti-aging cosmetics, among others. Fungi have been highlighted in production of metabolites with biological activity, such as antioxidant. The main objective of this study was to select a fungus from resources of Brazilians Pampa and Atlantic Forest biomes with hability for production of antioxidants by submerged fermentation. The scavenging activity of the compounds was determined against radical 2.2-diphenyl-1-picrylhydrazyl (DPPH), superoxide and hydroxyl. Botryosphaeria dothidea exhibited higher potential for the production of antioxidants, and its supernatant showed percentage inhibition values of 94.47, 94.87 and 89.78% against DPPH, superoxide and hydroxyl radicals and IC50 of 0.206 mg/mL. The identification of the volatile compounds present in the broth verified the presence of the hexahydropyrrolizin-3-one; 1.2-benzenedicarboxylic acid bis(2-methylpropyl) ester and 3.6-bis(2-methylpropyl)-2.5-piperazinedione. According to this study, Botryosphaeria dothidea presents great potential to produce compounds with antioxidant activity.



Similar content being viewed by others
References
Halliwell B, Gutteridge JMC (2015) Free radicals in biology and medicine. Oxford University Press, Oxford
Mancini A, Meucci E, Bianchi A et al (2006) Antioxidant systems in human seminal plasma: Physiopathological meaning and new perspectives. In: Panglossi HV (ed) Antioxidants: new research. Nova Science Publishers, Nova Iorque
Halliwell B (2002) Food-derived antioxidants: how to evaluate their importance in food and in vivo. In: Cadenas E, Packer L Handbook of antioxidants. Nova Iorque, Marcel Dekker
Scotti L, Scotti MT, Cardoso C et al (2007) Molecular modeling applied to the development of molecules with antioxidant activity for cosmetic use. Rev Bras Cienc Farm 43:153–166
Kusari S, Hertweck C, Spiteller M (2012) Chemical ecology of endophytic fungi: origins of secondary metabolites. Chem Biol 19:792–798
Silva IP (2014) Endophytic fungi: source alternative to secondary metabolites of plants. Enciclopédia Biosfera 10:3888–3905
Zhao J, Ma D, Luo M et al (2014) In vitro antioxidant activities and antioxidant enzyme activities in HepG2 cells and main active compounds of endophytic fungus from pigeon pea [Cajanus cajan (L.) Millsp.]. Food Res Int 56:243–251
Huang W, Cai Y, Xing J et al (2007) A potential antioxidant resource: endophytic fungi from medicinal plants. Econ Bot 61:14–30
Uma Anitha KPG, Mythili S (2017) Antioxidant and hepatoprotective potencials of novel endophytic fungus Achaetomium sp., from Euphorbia hirta. Asian Pac J Trop Med 10:588–593
Prihantini AI, Tachibana S (2017) Antioxidant compounds produced by Pseudocercospora sp. ESL 02, an endophytic fungus isolated from Elaeocarpus sylvestris. Asian Pac J Trop Biomed 7:110–115
Mahapatra S, Banerjee D (2013) Evaluation of in vitro antioxidant potency of exopolysaccharide from endophytic Fusarium solani SD5. Int J Biol Macromol 53:62–66
Brazil (2016) Ministry of the Environment.Atlantic forest, Pampa. https://www.mma.gov.br. Accessed 15 Jun 2016
Alfenas AC, Mafia RG (2007) Métodos em Fitopatologia. Viçosa, Ed. UFV
Souza ARC, Baldoni DB, Lima J et al (2015) Bioherbicide production by Diaporthe sp. isolated from the Brazilian Pampa biome. Biocatal Agric Biotechnol 4:575–578
Parra R, Aldred D, Magan N (2005) Medium optimization for the production of the secondary metabolite squalestatin S1 by a Phoma sp. combining orthogonal design and response surface methodology. Enzyme Microb Technol 37:704–711
Penariol MC, Monteiro AC, Pitelli RA (2008) Growth and sporulation of Bipolaris euphorbiae cultivated under different nutritional conditions. Cienc Rural 38:1907–1913
Pimentel FA, Cardoso MG, Batista RL et al (2010) Fungitoxic action of the essential oil of Tanaecium nocturnum (Barb. Rodr.) Bur. and K. Shum on Aspergillus flavus isolated from the Brazil nut (Bertholletia excelsa). Acta Amaz 40:213–220
Dal Prá V, Dolwitsch CB, Lima FO et al (2015) Ultrasound-assisted extraction and biological activities of Extracts of Brassica oleracea var. capitata. Food Technol Biotechnol 53:102–109
Alves CQ, David JM, David JP et al (2007) Methods for determination of in vitro antioxidant activity for extracts and organic compounds. Quím Nova 33(10):2202–2210
Sousa CMM, Silva HR, Vieira-Jr GM et al (2007) Total phenolics and antioxidant activity of five medicinal plants. Quím Nova 30:351–355
Zhao H, Dong J, Lu J et al (2006) Effects of extraction solvent mixtures on antioxidant activity evaluation and their extraction capacity and selectivity for free phenolic compounds in barley (Hordeum vulgare L.). J Agric Food Chem 54:7277–7286
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
White TJ, Bruns TD, Lee S et al (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninski JJ (eds) PCR protocols: a guide to methods and applications.Academic Press Inc, Nova Iorque
O’Donnell K, Cigelnik E (1997) Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol Phylogenetics Evol 7:103–116
Schmitz A, Riesner D (2006) Purification of nucleic acids by selective precipitation with polyethylene glycol 6000. Anal Biochem 354:311–313
Grune T, Schröder P, Biesalski HK (2005) Low molecular weight antioxidants. In: Grune T (ed) Oxidants and antioxidante defense systems: the handbook of environmental chemistry.Springer Berlin, Heidelberg, Berlin
Kikugawa K, Kunugi A, Kurechi T (1990) Chemistry and implications of degradation of phenolic antioxidants. In: Hudson BJF (ed) Food antioxidants. Elsevier Applied Science, London
Li XJ, Zhang Q, Zhang AL et al (2012) Metabolites from Aspergillus fumigatus, an endophytic fungus associated with Melia azedarach, and their antifungal, antifeedant and toxic activities. J Agric Food Chem 60:3424–3431
Zhang Q, Wang SQ, Tang HY et al (2013) Potential allelopathic indole diketopiperazines produced by the plant endophytic Aspergillus fumigatus using the one strain−many compounds method. J Agric Food Chem 61:11447–11452
Xiao J, Zhang Q, Gao YQ et al (2014) Antifungal and antibacterial metabolites from an endophytic Aspergillus sp. associated with Melia azedarach. Nat Prod Res 28:1388–1392
Yuan Y, Tian JM, Xiao J et al (2014) Bioactive metabolites isolated from Penicillium sp. YY-20, the endophytic fungus from Ginkgo biloba. Nat Prod Res 28:278–281
Zeng PY, Wu JG, Liao LM et al (2011) In vitro antioxidant activities of endophytic fungi isolated from the liverwort Scapania verrucosa. Genet Mol Res 10:3169–3179
Khiralla A, Mohamed I, Thomas J et al (2015) A pilot study of antioxidant potential of endophytic fungi from some Sudanese medicinal plants. Asian Pac J Trop Med 8:701–704
Jha DK, Panda L, Ramaiah S et al (2014) Evaluation and comparison of radical scavenging properties of solvent extracts from Justicia adhatoda leaf using DPPH assay. Appl Biochem Biotechnol 174:2413–2425
Xiao J, Zhang Q, Gao YQ et al (2014) Secondary metabolites from the endophytic Botryosphaeria dothidea of Melia azedarach and their antifungal, antibacterial, antioxidant, and cytotoxic activities. J Agric Food Chem 62:3584–3590
Amaral L (1995) A química. Edições Loyola, São Paulo
Huang Y, Suzuki S, Liu G et al (2011) Asymmetric synthesis of chiral trifluoromethylated heliotridane via highly catalytic asymmetric Friedel-Crafts alkylation with β-trifluoromethylated acrylates and pyrroles. New J Chem 35:2614–2621
Marcano D, Hasegawa M (2002) Fitoquímica orgánica. Editorial Torino, Caracas
Gao H, Song Y, Lv C et al (2015) The possible allelopathic effect of Hydrilla verticillata on phytoplankton in nutrient-rich water. Environ Earth Sci 73:5141–5151
Li X, Zhang J, Gao W et al (2012) Study on chemical composition, anti-inflammatory and anti-microbial activities of extracts from Chinese pear fruit (Pyrus bretschneideri Rehd.). Food Chem Toxicol 50:3673–3679
Deng HY, Xing JG, Luo DQ (2011) Metabolites of endophytic fungus Pestalotiopsis clavispora isolated from the stem of Bruguiera sexangula. Mycosystema 30:263–267
Zheng L, Yan X, Xu J et al (2005) Hymeniacidon perleve associated bioactive bacterium Pseudomonas sp. NJ6-3-1. Appl Biochem Microbiol 41:29–33
Balachandran C, Duraipandiyan V, Ignacimuthu S (2012) Cytotoxic (A549) and antimicrobial effects of Methylobacterium sp. isolate (ERI-135) from Nilgiris forest soil, India. Asian Pac J Trop Biomed 2(9):712–716
Auto HF, Constant JMC, Constant ABL (2008) Antibióticos e quimioterápicos. UFAM, Maceió
Acknowledgements
The authors would like to thank to CAPES (Coordination for the Improvement of Higher Education Personnel) by the scholarship for the author S. P. D.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Druzian, S.P., Pinheiro, L.N., Susin, N.M.B. et al. Production of metabolites with antioxidant activity by Botryosphaeria dothidea in submerged fermentation. Bioprocess Biosyst Eng 43, 13–20 (2020). https://doi.org/10.1007/s00449-019-02200-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-019-02200-y