Abstract
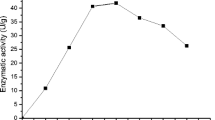
Inulinases are used for the production of high-fructose syrup and fructooligosaccharides, and are widely utilized in food and pharmaceutical industries. In this study, different carbon sources were screened for inulinase production by Aspergillus niger in shake flask fermentation. Optimum working conditions of the enzyme were determined. Additionally, some properties of produced enzyme were determined [activation (Ea)/inactivation (Eia) energies, Q10 value, inactivation rate constant (kd), half-life (t1/2), D value, Z value, enthalpy (ΔH), free energy (ΔG), and entropy (ΔS)]. Results showed that sugar beet molasses (SBM) was the best in the production of inulinase, which gave 383.73 U/mL activity at 30 °C, 200 rpm and initial pH 5.0 for 10 days with 2% (v/v) of the prepared spore solution. Optimum working conditions were 4.8 pH, 60 °C, and 10 min, which yielded 604.23 U/mL, 1.09 inulinase/sucrase ratio, and 2924.39 U/mg. Additionally, Ea and Eia of inulinase reaction were 37.30 and 112.86 kJ/mol, respectively. Beyond 60 °C, Q10 values of inulinase dropped below one. At 70 and 80 °C, t1/2 of inulinase was 33.6 and 7.2 min; therefore, inulinase is unstable at high temperatures, respectively. Additionally, t1/2, D, ΔH, ΔG values of inulinase decreased with the increase in temperature. Z values of inulinase were 7.21 °C. Negative values of ΔS showed that enzymes underwent a significant process of aggregation during denaturation. Consequently, SBM is a promising carbon source for inulinase production by A. niger. Also, this is the first report on the determination of some properties of A. niger A42 (ATCC 204,447) inulinase.






Similar content being viewed by others
References
Kaur N, Gupta AK (2002) Applications of inulin and oligofructose in health and nutrition. J Biosci 27:703–714
Ronkart S, Blecker C, Fougnies C, Van Herck J, Wouters J, Paquot M (2006) Determination of physical changes of inulin related to sorption isotherms: An X-ray diffraction, modulated differential scanning calorimetry and environmental scanning electron microscopy study. Carbohyd Polym 63:210–217
Chi Z-M, Zhang T, Cao T-S, Liu X-Y, Cui W, Zhao C-H (2011) Biotechnological potential of inulin for bioprocesses. Biores Technol 102:4295–4303
Singh RS, Chauhan K, Kennedy JF (2016) A panorama of bacterial inulinases: production, purification, characterization and industrial applications. Int J Biol Macromol 96:312–322
Singh P, Gill PK (2006) Production of inulinases: recent advances. Food Technol Biotechnol 44:151–162
Pandey A, Soccol CR, Selvakumar P, Soccol VT, Krieger N, Fontana JD (1999) Recent developments in microbial inulinases. Appl Biochem Biotechnol 81:35–52
Vandamme EJ, Derycke DG (1983) Microbial inulinases: fermentation process, properties, and applications. Adv Appl Microbiol 29:139–176
Kango N, Jain SC (2011) Production and properties of microbial inulinases: recent advances. Food Biotechnol 25:165–212
Ohta K, Akimoto H, Moriyama S (2004) Fungal inulinases: enzymology, molecular biology and biotechnology. J Appl Glycosci 51:247–254
Singh R, Singh R (2017) Inulinases. In: Current developments in biotechnology and bioengineering: production, isolation and purification of industrial products. Elsevier, Amsterdam, pp 423–446
Singh RS, Chauhan K, Pandey A, Larroche C, Kennedy JF (2018) Purification and characterization of two isoforms of exoinulinase from Penicillium oxalicum BGPUP-4 for the preparation of high fructose syrup from inulin. Int J Biol Macromol 118:1974–1983
Turhan I, Bialka KL, Demirci A, Karhan M (2010) Ethanol production from carob extract by using Saccharomyces cerevisiae. Biores Technol 101:5290–5296
Ongen-Baysal G, Sukan SS, Vassilev N (1994) Production and properties of inulinase from Aspergillus niger. Biotech Lett 16:275–280
Cemeroğlu B (2015) Reaksiyon kinetiği. Bizim Grup Basımevi, Ankara
Pal A, Khanum F (2011) Characterizing and improving the thermostability of purified xylanase from Aspergillus niger DFR-5 grown on solid-state-medium. J Biochem Technol 2:203–209
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Bender JP, Mazutti MA, de Oliveira D, Di Luccio M, Treichel H (2006) Inulinase production by Kluyveromyces marxianus NRRL Y-7571 using solid state fermentation. Appl Biochem Biotechnol 132:951–958
Kalil S, Suzan R, Mougeri F, Rodrigues M (2001) Optimization of inulinase production by Kluyveromyces marxianus using factorial design. Appl Biochem Biotechnol 94:257–264
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Ettalibi M, Baratti JC (1987) Purification, properties and comparison of invertase, exoinulinases and endoinulinases of Aspergillus ficuum. Appl Microbiol Biotechnol 26:13–20
de Oliveira Lino FS, Basso TO, Sommer MOA (2018) A synthetic medium to simulate sugarcane molasses. Biotechnol Biofuels 11:221
Demirci A, Öziyci HR, Karhan M, Turkenburg JP (2014) Fermentasyon besiyeri. In: Turhan I (ed) Endüstriyel mikrobiyolojiye giriş. Palme Yayıncılık, Ankara
Yuan X-L, Goosen C, Kools H, van der Maarel MJEC, van den Hondel CAMJJ, Dijkhuizen L, Ram AFJ (2006) Database mining and transcriptional analysis of genes encoding inulin-modifying enzymes of Aspergillus niger. Microbiology 152:3061–3073
Grootwassink JWD, Hewitt GM (1983) Inducible and constitutive formation of -fructofuranosidase (inulase) in batch and continuous cultures of the yeast Kluyveromyces fragilis. Microbiology 129:31–41
Dinarvand M, Ariff BA, Moeini H, Masomian M, Mousavi SS, Nahavandi R, Mustafa S (2012) Effect of extrinsic and intrinsic parameters on inulinase production by Aspergillus niger ATCC 20611. Electron J Biotechnol 15:5
Saber W, El-Naggar NE (2009) Optimization of fermentation conditions for the biosynthesis of inulinase by the new source; Aspergillus tamarii and hydrolysis of some inulin containing agro-wastes. Biotechnology 8:425–433
Makino Y, Treichel H, Mazutti MA, Maugeri F, Rodrigues MI (2009) Inulinase bio-production using agroindustrial residues: screening of microorganisms and process parameters optimization. J Chem Technol Biotechnol 84:1056–1062
Shuler ML, Kargi F, DeLisa M (2017) Bioprocess engineering: basic concepts, 3rd edn. Prentice Hall Upper Saddle River, NJ
Li Q, Coffman AM, Ju L-K (2015) Development of reproducible assays for polygalacturonase and pectinase. Enzyme Microbial Technol 72:42–48
Silva MF, Rigo D, Mossi V, Dallago RM, Henrick P, de Oliveira Kuhn G, Dalla Rosa C, Oliveira D, Oliveira JV, Treichel H (2013) Evaluation of enzymatic activity of commercial inulinase from Aspergillus niger immobilized in polyurethane foam. Food Bioprod Process 91:54–59
Poorna V, Kulkarni P (1995) A study of inulinase production in Aspergillus niger using fractional factorial design. Biores Technol 54:315–320
Yewale T, Singhal RS, Vaidya AA (2013) Immobilization of inulinase from Aspergillus niger NCIM 945 on chitosan and its application in continuous inulin hydrolysis. Biocatal Agric Biotechnol 2:96–101
Laowklom N, Chantanaphan R, Pinphanichakarn P (2012) Production, purification and characterization of inulinase from a newly isolated Streptomyces sp. CP01. Nat Resour 3:137
Naidoo K, Ayyachamy M, Permaul K, Singh S (2009) Enhanced fructooligosaccharides and inulinase production by a Xanthomonas campestris pv. phaseoli KM 24 mutant. Bioprocess Biosyst Eng 32:689–695
Sheng J, Chi Z, Gong F, Li J (2008) Purification and characterization of extracellular inulinase from a marine yeast Cryptococcus aureus G7a and inulin hydrolysis by the purified inulinase. Appl Biochem Biotechnol 144:111–121
Sheng J, Chi Z, Yan K, Wang X, Gong F, Li J (2009) Use of response surface methodology for optimizing process parameters for high inulinase production by the marine yeast Cryptococcus aureus G7a in solid-state fermentation and hydrolysis of inulin. Bioprocess Biosyst Eng 32:333–339
Canli O, Tasar GE, Taskin M (2013) Inulinase production by Geotrichum candidum OC-7 using migratory locusts as a new substrate and optimization process with Taguchi DOE. Toxicol Ind Health 29:704–710
Liu G-L, Fu G-Y, Chi Z, Chi Z-M (2014) Enhanced expression of the codon-optimized exo-inulinase gene from the yeast Meyerozyma guilliermondii in Saccharomyces sp. W0 and bioethanol production from inulin. Appl Microbiol Biotechnol 98:9129–9138
Singh R, Dhaliwal R, Puri M (2006) Production of inulinase from Kluyveromyces marxianus YS-1 using root extract of Asparagus racemosus. Process Biochem 41:1703–1707
Jain SC, Jain P, Kango N (2012) Production of inulinase from Kluyveromyces marxianus using Dahlia tuber extract. Braz J Microbiol 43:62–69
Kalil SJ, Suzan R, Maugeri F, Rodrigues MI (2001) Optimization of inulinase production by Kluyveromyces marxianus using factorial design. Appl Biochem Biotechnol 94:257–264
Gong F, Sheng J, Chi Z, Li J (2007) Inulinase production by a marine yeast Pichia guilliermondii and inulin hydrolysis by the crude inulinase. J Ind Microbiol Biotechnol 34:179–185
Silva-Santisteban BOY, Converti A, Maugeri Filho F (2009) Effects of carbon and nitrogen sources and oxygenation on the production of inulinase by Kluyveromyces marxianus. Appl Biochem Biotechnol 152:249–261
Gou Y, Li J, Zhu J, Xu W, Gao J (2015) Enhancing inulinase yield by irradiation mutation associated with optimization of culture conditions. Braz J Microbiol 46:911–920
Gong F, Zhang T, Chi Z, Sheng J, Li J, Wang X (2008) Purification and characterization of extracellular inulinase from a marine yeast Pichia guilliermondii and inulin hydrolysis by the purified inulinase. Biotechnol Bioprocess Eng 13:533–539
Yuan B, Hu N, Sun J, Wang S-A, Li F-L (2012) Purification and characterization of a novel extracellular inulinase from a new yeast species Candida kutaonensis sp. nov. KRF1T. Appl Microbiol Biotechnol 96:1517–1526
Zhou J, Peng M, Zhang R, Li J, Tang X, Xu B, Ding J, Gao Y, Ren J, Huang Z (2015) Characterization of Sphingomonas sp. JB13 exo-inulinase: a novel detergent-, salt-, and protease-tolerant exo-inulinase. Extremophiles 19:383–393
Hu N, Yuan B, Sun J, Wang S-A, Li F-L (2012) Thermotolerant Kluyveromyces marxianus and Saccharomyces cerevisiae strains representing potentials for bioethanol production from Jerusalem artichoke by consolidated bioprocessing. Appl Microbiol Biotechnol 95:1359–1368
Wang D, Li F-L, Wang S-A (2016) Engineering a natural Saccharomyces cerevisiae strain for ethanol production from inulin by consolidated bioprocessing. Biotechnol Biofuels 9:96. https://doi.org/10.1186/s13068-016-0511-4
Sirisansaneeyakul S, Worawuthiyanan N, Vanichsriratana W, Srinophakun P, Chisti Y (2007) Production of fructose from inulin using mixed inulinases from Aspergillus niger and Candida guilliermondii. World J Microbiol Biotechnol 23:543–552
Gao J, Yuan W, Li Y, Xiang R, Hou S, Zhong S, Bai F (2015) Transcriptional analysis of Kluyveromyces marxianus for ethanol production from inulin using consolidated bioprocessing technology. Biotechnol Biofuels 8:1
Chen G-J, Yang J-K, Peng X-B, He J-R (2016) High-level secretory expression of Aspergillus exo-inulinase and its use in the preparation of fructose syrup from inulin. J Mol Catal B Enzym 133:S543–S551
Torabizadeh H, Habibi-Rezaei M, Safari M, Moosavi-Movahedi AA, Sharifizadeh A, Azizian H, Amanlou M (2011) Endo-inulinase stabilization by pyridoxal phosphate modification: a kinetics, thermodynamics, and simulation approach. Appl Biochem Biotechnol 165:1661–1673
Gill PK, Manhas RK, Singh P (2006) Purification and properties of a heat-stable exoinulinase isoform from Aspergillus fumigatus. Biores Technol 97:894–902
Flores-Gallegos AC, Contreras-Esquivel JC, CbN Aguilar (2015) Comparative study of fungal strains for thermostable inulinase production. J Biosci Bioeng 119:421–426
Tanaka A, Hoshino E (2002) Calcium-binding parameter of Bacillus amyloliquefaciens α-amylase determined by inactivation kinetics. Biochem J 364:635–639
Marin E, Sanchez L, Perez M, Puyol P, Calvo M (2003) Effect of heat treatment on bovine lactoperoxidase activity in skim milk: kinetic and thermodynamic analysis. J Food Sci 68:89–93
Anema SG, McKenna AB (1996) Reaction kinetics of thermal denaturation of whey proteins in heated reconstituted whole milk. J Agric Food Chem 44:422–428
Acknowledgements
This study was supported by the Akdeniz University Research Foundation [Grant number: FDK-2019-4761].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors in this study mutually agree for submitting our manuscript to Biochemical Engineering Journal and declare that they have no conflict of interest in the publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Germec, M., Turhan, I. Evaluation of carbon sources for the production of inulinase by Aspergillus niger A42 and its characterization. Bioprocess Biosyst Eng 42, 1993–2005 (2019). https://doi.org/10.1007/s00449-019-02192-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-019-02192-9