Abstract
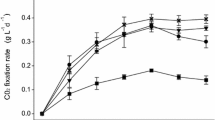
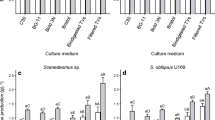
Biogas, a gaseous effluent from the anaerobic digestion of organic waste, is considered an important source of energy, since it has a composition mainly of methane (CH4; 55–75%) and CO2 (20–60%). Today, CO2 from biogas is an excellent carbon source to induce high microalgal biomass production; however, each microalga strain can have different optimal CO2 concentrations for maximizing their bio-refinery capacity as well as different ability to endure stressful conditions of industrial effluents. This study assessed the bio-refinery capacity of Chlorella sp. and Scenedesmus sp., native of Lago de Chapala, Mexico, from biogas, as well as the effect of high CO2 and methane concentrations on the physiological performance to grow, capture CO2 and biochemical composition of both microalgae cultured under different biogas compositions. The results show that both microalgae have the biotechnological potential to endure biogas compositions of 25% CO2–75% CH4. Under this condition, the biomass production attained by Chlorella sp. and Scenedesmus sp. was 1.77 ± 0.32 and 2.25 ± 0.20 g L−1, respectively, with a biochemical composition mainly of carbohydrates and proteins. Overall, this study demonstrates that both microalgae have the ability to endure the stressful biogas composition without affecting their physiological capacity to capture CO2 and biosynthesize high-value metabolites. Moreover, it is worth highlighting the importance of screening wild-type microalgae from local ecosystems to determine their physiological capacity for each biotechnological application.




Similar content being viewed by others
References
Yadav G, Sen R (2017) Microalgal green refinery concept for biosequestration of carbon-dioxide vis-à-vis wastewater remediation and bioenergy production: recent technological advances in climate research. J CO2 Util 17:188–206. https://doi.org/10.1016/j.jcou.2016.12.006
Rizwan M, Mujtaba G, Memon SA, Lee K, Rashid N (2018) Exploring the potential of microalgae for new biotechnology applications and beyond: a review. Renew Sustain Energy Rev 92:394–404. https://doi.org/10.1016/j.rser.2018.04.034
Chen Y-d, Ho S-H, Nagarajan D, Ren N-q, Chang J-S (2018) Waste biorefineries—integrating anaerobic digestion and microalgae cultivation for bioenergy production. Curr Opin Biotechnol 50:101–110. https://doi.org/10.1016/j.copbio.2017.11.017
Kao CY, Chiu SY, Huang TT, Dai L, Hsu L-K, Lin C-S (2012) Ability of a mutant strain of the microalga Chlorella sp. to capture carbon dioxide for biogas upgrading. Appl Energy 93:176–183. https://doi.org/10.1016/j.apenergy.2011.12.082
Van Der Ha D, Nachtergaele L, Kerckhof FM, Rameiyanti D, Bossier P, Verstraete W, Boon N (2012) Conversion of biogas to bioproducts by algae and methane oxidizing bacteria. Environ Sci Technol 46:13425–13431. https://doi.org/10.1021/es303929s
Meier L, Pérez R, Azócar L, Rivas M, Jeison D (2015) Photosynthetic CO2 uptake by microalgae: an attractive tool for biogas upgrading. Biomass Bioenergy 73:102–109. https://doi.org/10.1016/j.biombioe.2014.10.032
Xu J, Zhao Y, Zhao G, Zhang H (2015) Nutrient removal and biogas upgrading by integrating freshwater algae cultivation with piggery anaerobic digestate liquid treatment. Appl Microbiol Biotechnol 99:6493–6501. https://doi.org/10.1007/s00253-015-6537-x
Prandini JM, da Silva MLB, Mezzari MP, Pirolli M, Michelon W, Soares HM (2016) Enhancement of nutrient removal from swine wastewater digestate coupled to biogas purification by microalgae Scenedesmus spp. Bioresour Technol 202:67–75. https://doi.org/10.1016/j.biortech.2015.11.082
Choix FJ, Polster E, Corona-González RI, Snell-Castro R, Méndez-Acosta HO (2017) Nutrient composition of culture media induces different patterns of CO2 fixation from biogas and biomass production by the microalga Scenedesmus obliquus U169. Bioprocess Biosyst Eng. https://doi.org/10.1007/s00449-017-1828-5
Cheah WY, Show PL, Chang J-S, Ling TC, Juan JC (2015) Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresour Technol 184:190–201. https://doi.org/10.1016/j.biortech.2014.11.026
Zhou W, Wang J, Chen P, Ji C, Kang Q, Lu B, Li K, Liu J, Ruan R (2017) Bio-mitigation of carbon dioxide using microalgal systems: advances and perspectives. Renew Sustain Energy Rev 76:1163–1175. https://doi.org/10.1016/j.rser.2017.03.065
Varshney P, Beardall J, Bhattacharya S, Wangikar PP (2018) Isolation and biochemical characterisation of two thermophilic green algal species-Asterarcys quadricellulare and Chlorella sorokiniana, which are tolerant to high levels of carbon dioxide and nitric oxide. Algal Res 30:28–37. https://doi.org/10.1016/j.algal.2017.12.006
Kao CY, Chiu SY, Huang TT, Dai L, Wang G-H, Tseng C-P, Chen C-H, Lin C-S (2012) A mutant strain of microalga Chlorella sp. for the carbon dioxide capture from biogas. Biomass Bioenergy 36:132–140. https://doi.org/10.1016/j.biombioe.2011.10.046
Ghosh A, Khanra S, Mondal M, Halder G, Tiwari ON, Saini S, Bhowmick TK, Gayen K (2016) Progress toward isolation of strains and genetically engineered strains of microalgae for production of biofuel and other value added chemicals: a review. Energy Convers Manag 113:104–118. https://doi.org/10.1016/j.enconman.2016.01.050
Rocha RP, Machado M, Vaz MGMV, Vinson CC, Leite M, Richard R, Mendes LBB, Araujo WL, Caldana C, Martins MA, Williams TCR, Nunes-nesi A (2017) Exploring the metabolic and physiological diversity of native microalgal strains (Chlorophyta) isolated from tropical freshwater reservoirs. Algal Res 28:139–150. https://doi.org/10.1016/j.algal.2017.10.021
Solovchenko A, Khozin-Goldberg I (2013) High-CO2 tolerance in microalgae: possible mechanisms and implications for biotechnology and bioremediation. Biotechnol Lett 35:1745–1752. https://doi.org/10.1007/s10529-013-1274-7
Vázquez-García JA, Mora-Navarro MR, Vargas-Rodríguez YL (2004) Ordination of phytoplankton communities in the Chapala Lake, Jalisco-Michoacan, Mexico. Hidrobiológica 14:91–103
Smith DL, Johnson KB, Darmstadt TU (1996) A guide to marine coastal plankton and marine invertebrate larvae, 2nd edn. Kendall/Hunt Publishing Company, Iowa
Tang D, Han W, Li P, Miao X, Zhong J (2011) CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour Technol 102:3071–3076. https://doi.org/10.1016/j.biortech.2010.10.047
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Papazi A, Makridis P, Divanach P, Kotzabasis K (2016) Bioenergetic changes in the microalgal photosynthetic apparatus by extremely high CO2 concentrations induce an intense biomass production. Physiol Plant 132:338–349. https://doi.org/10.1111/j.1399-3054.2007.01015.x
Thiansathit W, Keener TC, Khang S (2015) The kinetics of Scenedesmus obliquus microalgae growth utilizing carbon dioxide gas from biogas. Biomass Bioenergy 76:79–85. https://doi.org/10.1016/j.biombioe.2015.03.012
Yan C, Zhang L, Luo X, Zheng Z (2014) Influence of influent methane concentration on biogas upgrading and biogas slurry purification under various LED (light-emitting diode) light wavelengths using Chlorella sp. Energy 69:419–426
Razzak SA, Hossain MM, Lucky RA, Bassi AS, de Lasa H (2013) Integrated CO2 capture, wastewater treatment and biofuel production by microalgae culturing—a review. Renew Sustain Energy Rev 27:622–653. https://doi.org/10.1016/j.rser.2013.05.063
Zhao B, Su Y (2014) Process effect of microalgal-carbon dioxide fixation and biomass production: a review. Renew Sustain Energy Rev 31:121–132. https://doi.org/10.1016/j.rser.2013.11.054
Markou G, Vandamme D, Muylaert K (2014) Microalgal and cyanobacterial cultivation: the supply of nutrients. Water Res 65:186–202. https://doi.org/10.1016/j.watres.2014.07.025
Choix FJ, Ochoa-Becerra MA, Hsieh-Lo M, Mondragón-Cortez P, Méndez-Acosta HO (2018) High biomass production and CO2 fixation from biogas by Chlorella and Scenedesmus microalga using tequila vinasses as culture medium. J Appl Phycol 30:2247–2258. https://doi.org/10.1007/s10811-018-1433-2
Li Y, Han D, Sommerfeld M, Hu Q (2011) Photosynthetic carbon partitioning and lipid production in the oleaginous microalga Pseudochlorococcum sp. (Chlorophyceae) under nitrogen-limited conditions. Bioresour Technol 102:123–129. https://doi.org/10.1016/j.biortech.2010.06.036
Choix FJ, Bashan Y, Mendoza A, de-Bashan LE (2014) Enhanced activity of ADP glucose pyrophosphorylase and formation of starch induced by Azospirillum brasilense in Chlorella vulgaris. J Biotechnol 177:22–34. https://doi.org/10.1016/j.jbiotec.2014.02.014
Cheng P, Wang J, Liu T (2015) Effect of cobalt enrichment on growth and hydrocarbon accumulation of Botryococcus braunii with immobilized biofilm attached cultivation. Bioresour Technol 177:204–208
Acknowledgements
Francisco J. Choix acknowledges CONACYT (National Council for Science and Technology) for the support under the Program-project 2517 Cátedras CONACYT and Diana Fischer for editorial services in English.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramos-Ibarra, J.R., Snell-Castro, R., Neria-Casillas, J.A. et al. Biotechnological potential of Chlorella sp. and Scenedesmus sp. microalgae to endure high CO2 and methane concentrations from biogas. Bioprocess Biosyst Eng 42, 1603–1610 (2019). https://doi.org/10.1007/s00449-019-02157-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-019-02157-y