Abstract
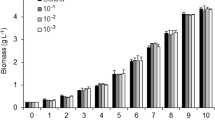
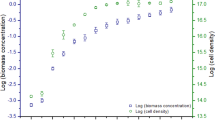
Environmental factors directly affect the growth and composition of microalgal biomass. Therefore, the present work analyzed the metabolomics (amino acids, organic acids, and fatty acids) of the microalga Scenedesmus obliquus cultivated in 24:0 and 12:12 (light:dark) photoperiods and different phases of cell growth. Furthermore, the metabolites were related to protein, lipid, and chlorophyll contents at the end of cultivation. The highest biomass concentration (4020 mg L− 1) and protein (47.3%) were obtained in culture under constant illumination. The cultivation 12:12 (light:dark) photoperiod triggered higher production of lipids (23.0%) and chlorophylls (26.4 mg g− 1) by S. obliquus. Microalgal metabolites were greatly affected by photoperiod and by phase of cell growth. Thus, metabolite production could be related to both the environmental conditions under which cultivation occurred and to the different concentrations of products (proteins, lipids, and chlorophylls) present in the S. obliquus biomass.



Similar content being viewed by others
References
Gavrisheva AI, Belokopytov BF, Semina VI, Shastik ES, Laurinavichene TV, Tsygankov AA (2015) Mass–energy balance analysis for estimation of light energy conversion in an integrated system of biological H2 production. Biofuel Res J 6:324–330
Yu X, Chen L, Zhang W (2015) Chemicals to enhance microalgal growth and accumulation of high-value bioproducts. Front Microbiol 6:56–65
Begum H, Yusoff FM, Banerjee S, Khatoon H, Shariff M (2016) Availability and utilization of pigments from microalgae. Crit Rev Food Sci Nutr 56(13):2209–2222
Skjånes K, Rebours C, Lindblad P (2013) Potential for green microalgae to produce hydrogen, pharmaceuticals and other high value products in a combined process. Crit Rev Biotechnol 33:172–215
Draaisma RB, Wijffels RH, Slegers PM, Brentner LB, Roy A, Barbosa MJ (2013) Food commodities from microalgae. Curr Opin Biotechnol 24:169–177
Mubarak M, Shaija A, Suchithra TV (2015) A review on the extraction of lipid from microalgae for biodiesel production. Algal Res 7:117–123
Wahidin S, Idris A, Shaleh SR (2013) The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresour Technol 129:7–11
Yan C, Zheng Z (2013) Performance of photoperiod and light intensity on biogas upgrade and biogas effluent nutrient reduction by the microalgae Chlorella sp. Bioresour Technol 139:292–299
Mohsenpour SF, Richards B, Willoughby N (2012) Spectral conversion of light for enhanced microalgae growth rates and photosynthetic pigment production. Bioresour Technol 125:75–81
Markou G, Nerantzis E (2013) Microalgae for high value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol Adv 31:1532–1542
Chen G, Zhao L, Qi Y (2015) Enhancing the productivity of microalgae cultivated in wastewater toward biofuel production: a critical review. Appl Energy 137:282–291
Gimpel JA, Henríquez V, Mayfield SP (2015) In metabolic engineering of eukaryotic microalgae: potential and challenges come with great diversity. Front Microbiol 6:1376–1389
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stainer RY (1979) Generic assignments strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111(1):1–61
APHA (1998) Standard methods for the examination of water and wastewater. American Public Health Association (APHA), Washington, DC
Horowitz W (2000) AOAC official methods of analysis. Association of Official Analytical Chemists International, Gaithersburg
Rodrigues DB, Menezes CR, Mercadante AZ, Jacob-Lopes E, Zepka LQ (2015) Bioactive pigments from microalgae Phormidium autumnale. Food Res Int 77(2):273–279
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate coefficients and simultaneous equations for assay chlorophyll a and b extracted with four different solvents: varification of the concentration of chlorophyll standards by atomic absorption spectrometry. Biochim Biophys Acta 975:384–394
Vendruscolo RG, Facchi MMX, Maroneze MM, Fagundes MB, Cichoski AJ, Zepka LQ, Barin JS, Jacob-Lopes E, Wagner R (2018) Polar and non-polar intracellular compounds from microalgae: Methods of simultaneous extraction, gas chromatography determination and comparative analysis. Food Res Int 109:204–212
Hartman L, Lago RCA (1973) Rapid preparation of fatty acids methyl esters. Lab Pract 22(6):475–476
Jacob-opes E, Scoparo CHG, Lacerda LMCF, Franco TT (2009) Effect of light cycles (night/day) on CO2 fixation and biomass production by microalgae in photobioreactors. Chem Eng Process 48(1):306–310
Krzemińska I, Pawlik-Skowrońska B, Trzcińska M, Tys J (2014) Influence of photoperiods on the growth rate and biomass productivity of green microalgae. Bioprocess Biosyst Eng 37:735–741
Ho SH, Chena CY, Yeha KL, Chenc WM, Lind CY, Chang JS (2010) Characterization of photosynthetic carbon dioxide fixation ability of indigenous Scenedesmus obliquus isolates. Biochem Eng J 53:57–62
Ma C, Zhang Y, Ho S, Xing D, Ren N, Liu B (2017) Cell growth and lipid accumulation of a microalgal mutant Scenedesmus sp. Z-4 by combining light/dark cycle with temperature variation. Biotechnol Biofuels 10:260–272
Hexin LV, Qu G, Qi X, Lu L, Tian C, Ma Y (2013) Transcriptome analysis of Chlamydomonas reinhardtii during the process of lipid accumulation. Genomics 101(4):229–237
George B, Pancha I, Desai C, Chokshi K, Paliwal C, Ghosh T, Mishra S (2014) Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus—a potential strain for bio-fuel production. Bioresour Technol 171:367–374
Seyfabadi J, Ramezanpour Z, Khoeyi ZA (2011) Protein, fatty acid, and pigment content of Chlorella vulgaris under different light regimes. J Appl Phycol 23:721–726
Chandra TS, Aditi S, Kumar MM, Mukherji S (2017) Growth and biochemical characteristics of an indigenous freshwater microalga, Scenedesmus obtusus, cultivated in an airlift photobioreactor: effect of reactor hydrodynamics, light intensity, and photoperiod. Bioprocess Biosyst Eng 40:1057–1068
Sharma R, Singh GP, Sharma VK (2012) Effects of culture conditions on growth and biochemical profile of Chlorella vulgaris. J Plant Pathol Microbiol 3:1–6
Oikawa A, Matsuda F, Kikuyama M, Mimura T, Saito K (2011) Metabolomics of a single vacuole reveals metabolic dynamism in an alga Chara australis. Plant Physiol 157:544–555
Gravot A, Dittami SM, Rousvoal S, Lugan R, Eggert A, Collén J, Boyen C, Bouchereau A, Tonon T (2010) Diurnal oscillations of metabolite abundances and gene analysis provide new insights into central metabolic processes of the brown alga Ectocarpus siliculosus. New Phytol 188:98–110
El-Sheekh MM, Fathy AA (2009) Variation of some nutritional constituents and fatty acid profiles of Chlorella vulgaris Beijerinck grown under auto and heterotrophic conditions. Inter J Botany 5:153–159
Khairy HM, Ali EM, Dowidar SM (2011) Comparative effects of autotrophic and heterotrophic growth on some vitamins, 2, 2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity, amino acids and protein profile of Chlorella vulgaris Beijerinck. Afr J Biotechnol 10:13514–13519
Ben-Izhak ME, Parola AH, Kost D (2003) Low-frequency electromagnetic fields induce a stress effect upon higher plants, as evident by the universal stress signal, alanine. Biochem Biophys Res Commun 302:427–434
Ben-Izhak ME, Levkovitz A, Kost D (2015) Ultraviolet radiation induces stress in etiolated Landoltia punctata, as evidenced by the presence of alanine, a universal stress signal: a 15N NMR study. Plant Biol 17:101–107
Nehmé R, Atieh C, Fayad S, Claude B, Chartier MT, Elleuch F, Abdelkafi S, Pichon C, Morin P (2017) Microalgae amino acid extraction and analysis at nanomolar level using electroporation and capillary electrophoresis with laser-induced fluorescence detection. J Sep Sci 40(2):558–566
Kang Z, Wang Y, Wang Q, Qi Q (2011) Metabolic engineering to improve 5-aminolevulinic acid production. Bioeng Bugs 2(6):342–345
Pereira DM, Valentão P, Andrade PB (2014) Marine natural pigments: chemistry, distribution and analysis. Dyes Pigm 111:124–134
Arnold AA, Genard B, Zito F, Tremblay R, Warschawski DE, Marcotte I (2015) Identification of lipid and saccharide constituents of whole microalgal cells by 13C solid-state NMR. Biochim Biophys Acta 1848:369–377
Demuez M, Mahdy A, Tomás-Pejó E, González-Fernández C, Ballesteros M (2015) Enzymatic cell disruption of microalgae biomass in biorefinery processes. Biotechnol Bioeng 112:1955–1966
Duan Z, Tan X, Guo J, Kahehu CW, Yang H, Zheng X, Zhu F (2017) Effects of biological and physical properties of microalgae on disruption induced by a low-frequency ultrasound. J Appl Phycol 29(6):2937–2946
Voigt J, Stolarczyk A, Zych M, Malec P, Burczyk J (2014) The predominant cell-wall polypeptide of Scenedesmus obliquus is related to the cell-wall glycoprotein gp3 of Chlamydomonas reinhardtii. Plant Sci 215–216:39–47
Hildebrand D (2010) Plant Biochemistry BCH/PPA/PLS 609. http://www.uky.edu/~dhild/biochem/24/lect24.html. Accessed 20 Mar 2018
Abomohra A, Wagner M, El-Sheekh M, Hanelt D (2013) Lipid and total fatty acid productivity in photoautotrophic fresh water microalgae: screening studies towards biodiesel production. J Appl Phycol 25(4):931–936
Tang H, Chen M, Garcia MED, Abunasser N, Simon Ng KY, Salley SO (2011) Culture of microalgae Chlorella minutissima for biodiesel feedstock production. Biotechnol Bioeng 108(10):2280–2287
Rismani-Yazdi H, Haznedaroglu BZ, Bibby K, Peccia J (2011) Transcriptome sequencing and annotation of the microalgae Dunaliella tertiolecta: pathway description and gene discovery for production of next-generation biofuels. BMC Genom 12:148–164
Talebi AF, Mohtashami SK, Tabatabaei M, Tohidfar M, Bagheri A, Zeinalabedini M, Mirzaei HH, Mirzajanzadeh M, Shafaroudi SM, Bakhtiari (2013) Fatty acids profiling: a selective criterion for screening microalgae strains for biodiesel production. Algal Res 2(3):258–267
Schmid M, Kraft LGK, van der Loos LM, Kraft GT, Virtue P, Nichols PD, Hurd CL (2018) Southern Australian seaweeds: a promising resource for omega-3 fatty acids. Food Chem 265:70–77
Acknowledgements
To the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vendruscolo, R.G., Fagundes, M.B., Maroneze, M.M. et al. Scenedesmus obliquus metabolomics: effect of photoperiods and cell growth phases. Bioprocess Biosyst Eng 42, 727–739 (2019). https://doi.org/10.1007/s00449-019-02076-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-019-02076-y