Abstract
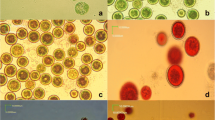
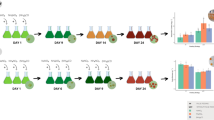
In the present study, the effects of four different culture media on the growth, astaxanthin production and morphology of Haematococcus pluvialis LUGU were studied under two-step cultivation. The interactions between astaxanthin synthesis and secondary messengers, reactive oxygen species (ROS) and mitogen-activated protein kinases (MAPK) were also investigated. In the first green vegetative cell stage, maximal biomass productivity (86.54 mg L−1 day−1) was obtained in BBM medium. In the induction stage, the highest astaxanthin content (21.5 mg g−1) occurred in BG-11 medium, which was higher than in any other media. The expressions of MAPK and astaxanthin biosynthetic genes in BG-11 were higher than in any other media, whereas the ROS content was lower. Biochemical and physiological analyses suggested that the ROS, MAPK and astaxanthin biosynthetic gene expression was involved in astaxanthin biosynthesis in H. pluvialis under different culture media conditions. This study proposes a two-step cultivation strategy to efficiently produce astaxanthin using microalgae.






Similar content being viewed by others
References
Guerin M, Huntley ME, Olaizola M (2003) Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol 21(5):210–216
Higuera-Ciapara I, Felix-Valenzuela L, Goycoolea FM (2006) Astaxanthin: a review of its chemistry and applications. Crit Rev Food Sci 46(2):185–196
Markou G, Nerantzis E (2013) Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol Adv 31(8):1532–1542
Li J, Zhu D, Niu J, Shen S, Wang G (2011) An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol Adv 29(6):568–574
Shah MMR, Liang Y, Cheng JJ, Daroch M (2016) Astaxanthin-producing green microalga Haematococcus pluvialis: from single cell to high value commercial products. Front Plant Sci 7:531
Scibilia L, Girolomoni L, Berteotti S, Alboresi A, Ballottari M (2015) Photosynthetic response to nitrogen starvation and high light in Haematococcus pluvialis. Algal Res 12:170–181
Hong ME, Hwang SK, Chang WS, Kim BW, Lee J, Sim SJ (2015) Enhanced autotrophic astaxanthin production from Haematococcus pluvialis under high temperature via heat stress-driven Haber–Weiss reaction. Appl Microbiol Biot 99(12):5203–5215
Sharma KK, Ahmed F, Schenk PM, Li Y (2015) UV-C mediated rapid carotenoid induction and settling performance of Dunaliella salina and Haematococcus pluvialis. Biotechnol Bioeng 112(10):2106–2114
Park JC, Choi SP, Hong ME, Sim SJ (2014) Enhanced astaxanthin production from microalga, Haematococcus pluvialis by two-stage perfusion culture with stepwise light irradiation. Bioprocess Biosyst Eng 37(10):2039–2047
Choi YE, Yun YS, Park JM, Yang JW (2011) Determination of the time transferring cells for astaxanthin production considering two-stage process of Haematococcus pluvialis cultivation. Bioresour Technol 102(24):11249–11253
Zhao Y, Li D, Ding K, Che R, Xu JW, Zhao P, Li T, Ma H, Yu X (2016) Production of biomass and lipids by the oleaginous microalgae Monoraphidium sp. QLY-1 through heterotrophic cultivation and photo-chemical modulator induction. Bioresour Technol 211:669–676
Fábregas J, Otero A, Maseda A, Dominguez A (2001) Two-stage cultures for the production of astaxanthin from Haematococcus pluvialis. J Biotechnol 89(1):65–71
Zhang Z, Wang B, Hu Q, Sommerfeld M, Li Y (2016) A new paradigm for producing astaxanthin from the unicellular green alga Haematococcus pluvialis. Biotechnol Bioeng 113(10):2088–2099
Shang M, Ding W, Zhao Y, Xu JW, Zhao P, Li T, Ma H, Yu X (2016) Enhanced astaxanthin production from Haematococcus pluvialis using butylated hydroxyanisole. J Biotechnol 236:199–207
Aflalo C, Meshulam Y, Zarka A, Boussiba S (2007) On the relative efficiency of two-vs. one-stage production of astaxanthin by the green alga Haematococcus pluvialis. Biotechnol Bioeng 98(1):300–305
George B, Pancha I, Desai C, Chokshi K, Paliwal C, Ghosh T, Mishra S (2014) Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus—a potential strain for bio-fuel production. Bioresour Technol 171:367–374
Rai MP, Gupta S (2017) Effect of media composition and light supply on biomass, lipid content and FAME profile for quality biofuel production from Scenedesmus abundans. Energy Convers Manag 141:85–92
Zhao Y, Li D, Xu JW, Zhao P, Ma H, Yu X (2018) Melatonin enhances lipid production in Monoraphidium sp. QLY-1 under nitrogen deficiency conditions via a multi-level mechanism. Bioresour Technol 259:46–53
Ding W, Zhao P, Peng J, Zhao Y, Xu JW, Li T, Reiter RJ, Ma H, Yu X (2018) Melatonin enhances astaxanthin accumulation in the green microalga Haematococcus pluvialis by mechanisms possibly related to abiotic stress tolerance. Algal Res 33:256–265
Moustafa K, AbuQamar S, Jarrar M, Al-Rajab AJ, Trémouillaux-Guiller J (2014) MAPK cascades and major abiotic stresses. Plant Cell Rep 33(8):1217–1225
Li Y, Sommerfeld M, Chen F, Hu Q (2008) Consumption of oxygen by astaxanthin biosynthesis: a protective mechanism against oxidative stress in Haematococcus pluvialis (Chlorophyceae). J Plant Physiol 165(17):1783–1797
Steinbrenner J, Linden H (2001) Regulation of two carotenoid biosynthesis genes coding for phytoene synthase and carotenoid hydroxylase during stress-induced astaxanthin formation in the green alga Haematococcus pluvialis. Plant Physiol 125(2):810–817
Grünewald K, Hirschberg J, Hagen C (2001) Ketocarotenoid biosynthesis outside of plastids in the unicellular green alga Haematococcus pluvialis. J Biol Chem 276(8):6023–6029
Kathiresan S, Chandrashekar A, Ravishankar GA, Sarada R (2015) Regulation of astaxanthin and its intermediates through cloning and genetic transformation of β-carotene ketolase in Haematococcus pluvialis. J Biotechnol 196:33–41
Zhao Y, Shang M, Xu JW, Zhao P, Li T, Yu X (2015) Enhanced astaxanthin production from a novel strain of Haematococcus pluvialis using fulvic acid. Process Biochem 50(12):2072–2077
Boussiba S, Vonshak A (1991) Astaxanthin accumulation in the green alga Haematococcus pluvialis. Plant Cell Physiol 32(7):1077–1082
Li D, Zhao Y, Ding W, Zhao P, Xu JW, Li T, Ma H, Yu X (2017) A strategy for promoting lipid production in green microalgae Monoraphidium sp. QLY-1 by combined melatonin and photoinduction. Bioresour Technol 235:104–112
Ding W, Zhao Y, Xu JW, Zhao P, Li T, Ma H, Reiter RJ, Yu X (2018) Melatonin: a multifunctional molecule that triggers defense responses against high light and nitrogen starvation stress in Haematococcus pluvialis. J Agric Food Chem 66(29):7701–7711
Kaewpintong K, Shotipruk A, Powtongsook S, Pavasant P (2007) Photoautotrophic high-density cultivation of vegetative cells of Haematococcus pluvialis in airlift bioreactor. Bioresour Technol 98(2):288–295
Singh P, Guldhe A, Kumari S, Rawat I, Bux F (2016) Combined metals and EDTA control: an integrated and scalable lipid enhancement strategy to alleviate biomass constraints in microalgae under nitrogen limited conditions. Energy Convers Manag 114:100–109
Kona R, Hemalatha M, Srivastav KV, Mohan SV (2017) Regulatory effect of Fe-EDTA on mixotrophic cultivation of Chlorella sp. towards biomass growth and metabolite production. Bioresour Technol 244:1227–1234
Ruangsomboon S, Sornchai P, Prachom N (2018) Enhanced hydrocarbon production and improved biodiesel qualities of Botryococcus braunii KMITL 5 by vitamins thiamine, biotin and cobalamin supplementation. Algal Res 29:159–169
Che R, Huang L, Yu X (2015) Enhanced biomass production, lipid yield and sedimentation efficiency by iron ion. Bioresour Technol 192:795–798
Wan M, Jin X, Xia J, Rosenberg JN, Yu G, Nie Z, Oyler GA, Betenbaugh MJ (2014) The effect of iron on growth, lipid accumulation, and gene expression profile of the freshwater microalga Chlorella sorokiniana. Appl Microbiol Biotechnol 98(22):9473–9481
Han D, Wang J, Sommerfeld M, Hu Q (2012) Susceptibility and protective mechanisms of motile and non motile cells of Haematococcus pluvialis (chlorophyceae) to photooxidative stress1. J Phycol 48(3):693–705
Sun Z, Cunningham FX, Gantt E (1998) Differential expression of two isopentenyl pyrophosphate isomerases and enhanced carotenoid accumulation in a unicellular chlorophyte. Proc Natl Acad Sci USA 95(19):11482–11488
Scherz-Shouval R, Elazar Z (2009) Monitoring starvation-induced reactive oxygen species formation. Method Enzymol 452:119–130
Gwak Y, Hwang Y, Wang B, Kim M, Jeong J, Lee CG, Hu Q (2014) Comparative analyses of lipidomes and transcriptomes reveal a concerted action of multiple defensive systems against photooxidative stress in Haematococcus pluvialis. J Exp Bot 65(15):4317–4334
Inzé D, Montagu M Van (1995) Oxidative stress in plants. Curr Opin Biotechnol 6(2):153–158
Asai S, Ohta K, Yoshioka H (2008) MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. Plant Cell 20(5):1390–1406
Zhao R, Ng DHP, Fang L, Chow YYS, Lee YK (2016) MAPK in Dunaliella tertiolecta regulates glycerol production in response to osmotic shock. Eur J Phycol 51(2):119–128
Raja V, Majeed U, Kang H, Andrabi KI, John R (2017) Abiotic stress: interplay between ROS, hormones and MAPKs. Environ Exp Bot 137:142–157
Li Y, Sommerfeld M, Chen F, Hu Q (2010) Effect of photon flux densities on regulation of carotenogenesis and cell viability of Haematococcus pluvialis (Chlorophyceae). J Appl Phycol 22(3):253–263
Ding W, Peng J, Zhao Y, Zhao P, Xu JW, Li T, Yu X (2018) A strategy for boosting astaxanthin accumulation in green microalga Haematococcus pluvialis by using combined diethyl aminoethyl hexanoate and high light. J Appl Phycol. https://doi.org/10.1007/s10811-018-1561-8
Acknowledgements
The National Natural Science Foundation of China (no. 21766012 to X. Yu), the Key Science and Technology Project of Yunnan Province, China (2018ZG003 to X. Yu), and the National Natural Science Foundation of China (no. 21666012 to P. Zhao) supported this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, Y., Yue, C., Geng, S. et al. Role of media composition in biomass and astaxanthin production of Haematococcus pluvialis under two-stage cultivation. Bioprocess Biosyst Eng 42, 593–602 (2019). https://doi.org/10.1007/s00449-018-02064-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-018-02064-8