Abstract
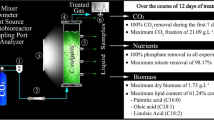
The research on microalgal biodiesel is focused not only on getting the highest lipid productivity but also desired quality of lipid. The experiments were initially conducted on flask scale (1L) using acetate carbon source at different concentrations viz. 0.5, 2, 3 and 4 g L−1. The optimum concentration of acetate was considered for further experiments in two airlift photobioreactors (10 L) equipped separately with red and white LED lights. The Feasibility Index (FI) was derived to analyze the scalability of mixotrophic cultivation based on net carbon fixation in biomass per consumption of total organic carbon. The experimental strategy under mixotrophic mode of cultivation lowered the α-linolenic acid content of lipid by 60–80% as compared to autotrophic cultivation for Scenedesmus abundans species and yielded the highest biomass and lipid productivities, 59 ± 2 and 17 ± 1.8 mg L−1 day−1, respectively. The TOC, nitrate, and phosphate reduction rates were 74.6 ± 3.0, 11.5 ± 1.4, 9.6 ± 2.4 mg L−1 day−1, respectively. The significant change was observed in lipid compositions due to the scale, mode of cultivation, and light spectra. As compared to phototrophic cultivation, biodiesel obtained under mixotrophic cultivation only met standard biodiesel properties. The FI data showed that the mixotrophic cultivation was feasible on moderate concentrations of acetate (2–3 g L−1).





Similar content being viewed by others
References
Gim GH, Kim JK, Kim HS, Kathiravan MN, Yang H, Jeong SH, Kim SW (2014) Comparison of biomass production and total lipid content of freshwater green microalgae cultivated under various culture conditions. Bioprocess Biosyst Eng 37:99–106
Apel AC, Weuster-Botz D (2015) Engineering solutions for open microalgae mass cultivation and realistic indoor simulation of outdoor environments. Bioprocess Biosyst Eng 38:995–108
Pawar S (2016) Effectiveness mapping of open raceway pond and tubular photobioreactors for sustainable production of microalgae biofuel. Renew Sustain Energy Rev 62:640–653
Li T, Zheng Y, Yu L, Chen S (2014) Mixotrophic cultivation of a Chlorella sorokiniana strain for enhanced biomass and lipid production. Biomass Bioenerg 66:204–213
Praveenkumar R, Kim B, Choi E, Lee K, Cho S, Hyun JS, Park JY, Lee YC, Lee HU, Lee JS, Oh YK (2014) Mixotrophic cultivation of oleaginous Chlorella sp. KR-1 mediated by actual coal-fired flue gas for biodiesel production. Bioprocess Biosyst Eng 37:2083–2094
Cheirsilp B, Torpee S (2012) Enhanced growth and lipid production of microalgae under mixotrophic culture condition: effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour Technol 110:510–516
Fei Q, Fu R, Shang L, Brigham CJ, Chang HN (2015) Lipid production by microalgae Chlorella protothecoides with volatile fatty acids (VFAs) as carbon sources in heterotrophic cultivation and its economic assessment. Bioprocess Biosyst Eng 38:691–700
Francisco EC, Neves DB, Lopes EJ, Franco TT (2010) Microalgae as feedstock for biodiesel production: carbon dioxide sequestration, lipid production and biofuel quality. J Chem Technol Biotechnol 85:395–403
Perez-Garcia O, Escalante FME, de-Bashan LE, Bashan Y (2011) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45:11–36
Vidyashankar S, Deviprasad K, Chauhan VS, Ravishankar GA, Sarada R (2013) Selection and evaluation of CO2 tolerant indigenous microalga Scenedesmus dimorphus for unsaturated fatty acid rich lipid production under different culture conditions. Bioresour Technol 144:28–37
Hoekman SK, Broch A, Robbins C, Ceniceros E, Natarajan M (2012) Review of biodiesel composition, properties, and specifications. Renew Sustain Energy Rev 16:143–169
Gupta S, Pandey R, Pawar S (2016) Microalgal bioremediation of food-processing industrial wastewater under mixotrophic conditions. Front Chem Sci Eng 10:499–508
Gupta S, Pandey R, Pawar S (2017) Bioremediation of synthetic high strength chemical oxygen demand wastewater using microalgae sp. Chlorella pyrenoidosa. Bioremediat J 21:38–51
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Anahas AMP, Muralitharan G (2015) Isolation and screening of heterocystous cyanobacterial strains for biodiesel production by evaluating the fuel properties from fatty acid methyl ester (FAME) profiles. Bioresour Technol 184:9–17
Krisnangkura KA (1986) Simple method for estimation of cetane index of vegetable oil methyl esters. J Am Oil Chem Soc 63:552–553
Ramos MJ, Fernandez CM, Casas A, Rodriguez L, Perez A (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100:261–268
Shen QH, Jiang JW, Chen LP, Cheng LH, Xu XH, Chen HL (2015) Effect of carbon source on biomass growth and nutrients removal of Scenedesmus obliquus for wastewater advanced treatment and lipid production. Bioresour Technol 190:257–263
Chapman SP, Paget CM, Johnson GN, Schwartz JM (2015) Flux balance analysis reveals acetate metabolism modulates cyclic electron flow and alternative glycolytic pathways in Chlamydomonas reinhardtii. Front Plant Sci 6:474. https://doi.org/10.3389/fpls.2015.00474
Liu X, Duan S, Li A, Xu N, Cai Z, Hu Z (2009) Effects of organic carbon sources on growth, photosynthesis, and respiration of Phaeodactylum tricornutum. J Appl Phycol 21:239–246
Krzeminska I, Oleszek M (2016) Glucose supplementation-induced changes in the Auxenochlorella protothecoides fatty acid composition suitable for biodiesel production. Bioresour Technol 218:1294–1297
Pawar SB (2016) Process engineering aspects of vertical gas sparged photobioreactors for mass production of microalgae. ChemBioEng Rev 3:101–115
Pawar SB (2017) Computational fluid dynamics (CFD) analysis of airlift bioreactor: effect of draft tube configurations on hydrodynamics, cell suspension, and shear rate. Bioprocess Biosyst Eng. https://doi.org/10.1007/s00449-017-1841-8
Baba M, Kikuta F, Suzuki I, Watanabe MM, Shiraiwa Y (2012) Wavelength specificity of growth, photosynthesis, and hydrocarbon production in the oil-producing green alga Botryococcus braunii. Bioresour Technol 109:266–270
Yan C, Zhao Y, Zheng Z, Luo X (2013) Effects of various LED light wavelengths and light intensity supply strategies on synthetic high-strength wastewater purification by Chlorella vulgaris. Biodegradation 24:721–732
Yan C, Luo X, Zheng Z (2013) Performance of purifying anaerobic fermentation slurry using microalgae in response to various LED light wavelengths and intensities. J Chem Technol Biotechnol 88:1622–1630
Edmundson SJ, Huesemann MH (2015) The dark side of algae cultivation: characterizing night biomass loss in three photosynthetic algae, Chlorella sorokiniana, Nannochloropsis salina and Picochlorum sp.. Algal Res 12:470–476
Gonzalez-Garcinuno A, Tabernero A, Sanchez-Alvarez JM, Martin del Valle EM, Galan MA (2014) Effect of nitrogen source on growth and lipid accumulation in Scenedesmus abundans and Chlorella ellipsoidea. Bioresour Technol 173:334–341
Wang Y, Chen T, Qin S (2013) Differential fatty acid profiles of Chlorella kessleri grown with organic materials. J Chem Technol Biotechnol 88:651–657
Acknowledgements
The corresponding author is thankful to the Department of Science and Technology, New Delhi for their financial support for this research work under the scheme of DST INSPIRE Faculty Award (IFA13-ENG63). Authors are very grateful to the Director of CSIR-NEERI Nagpur and Dr. H. J. Purohit, Head, EBGD, CSIR-NEERI for his continual support to enhance the quality of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gupta, S., Pawar, S.B. Mixotrophic cultivation of microalgae to enhance the quality of lipid for biodiesel application: effects of scale of cultivation and light spectrum on reduction of α-linolenic acid. Bioprocess Biosyst Eng 41, 531–542 (2018). https://doi.org/10.1007/s00449-017-1888-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-017-1888-6