Abstract
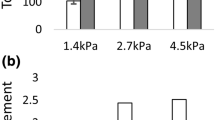
We study the adhesion limit of 3T6 fibroblasts, cultured in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum at 37 °C and 5% CO2, with a narrow-gap rotational rheometer in the parallel-disk configuration. Reducing the uncertainty in gap width to about 1 µm allows studying the cells at narrow gaps, which enables to study the critical shear stress of the cells in low-viscous media. The adhesion limit on fibronectin-coated glass plates is determined as a function of concentration and adhesion time. We found that cells in groups have a tendency to detach at slightly higher shear stresses than single cells. Moreover, 60 min after the settling phase are enough for the cells to adhere to the coated plate at maximum strength. We show that the setup may also be used for cells that are not adhered from suspension, but are grown directly on the substrate.






Similar content being viewed by others
References
Ahmad Khalili A, Ahmad MR, Tseng F-G (2015) A Review of cell adhesion studies for biomedical and biological applications. Int J Mol Sci 16(8):18149–18184. https://doi.org/10.3390/ijms160818149
Lee GYH, Lim CT (2007) Biomechanics approaches to studying human diseases. Trends Biotechnol 25(3):111–118. https://doi.org/10.1016/j.tibtech.2007.01.005
Christ KV, Williamson KB, Masters KS, Turner KT (2010) Measurement of single-cell adhesion strength using a microfluidic assay. Biomed Microdevice 12(3):443–455. https://doi.org/10.1007/s10544-010-9401-x
Palmer CP, Mycielska ME, Burcu H, Osman K, Collins T, Beckerman R, Perrett R, Johnson H, Aydar E, Djamgoz MBA (2008) Single cell adhesion measuring apparatus (SCAMA): application to cancer cell lines of different metastatic potential and voltage-gated Na + channel expression. Eur Biophys J 37(4):359–368. https://doi.org/10.1007/s00249-007-0219-2
Christ K, Turner K (2011) Methods to measure the strength of cell adhesion to substrates. Surface and interfacial aspects of cell adhesion. CRC Press, pp 193–224
Castelain M, Rouxhet PG, Pignon F, Magnin A, Piau J-M (2012) Single-cell adhesion probed in-situ using optical tweezers: a case study with Saccharomyces cerevisiae. J Appl Phys 111(11):114701. https://doi.org/10.1063/1.4723566
Channavajjala LS, Eidsath A, Saxinger WC (1997) A simple method for measurement of cell-substrate attachment forces: application to HIV-1 Tat. J Cell Sci 110(2):249
Reutelingsperger CPM, Van Gool RGJ, Heijnen V, Frederik P, Lindhout T (1994) The rotating disc as a device to study the adhesive properties of endothelial cells under differential shear stresses. J Mater Sci Mater Med 5(6):361–367. https://doi.org/10.1007/BF00058964
Goldstein AS, DiMilla PA (1997) Application of fluid mechanic and kinetic models to characterize mammalian cell detachment in a radial-flow chamber. Biotechnol Bioeng 55(4):616–629. https://doi.org/10.1002/(SICI)1097-0290(19970820)55:4<616::AID-BIT4>3.0.CO;2-K
Visser Claas W, Gielen Marise V, Hao Z, Le Gac S, Lohse D, Sun C (2015) Quantifying cell adhesion through impingement of a controlled microjet. Biophys J 108(1):23–31. https://doi.org/10.1016/j.bpj.2014.10.071
Lu H, Koo LY, Wang WM, Lauffenburger DA, Griffith LG, Jensen KF (2004) Microfluidic shear devices for quantitative analysis of cell adhesion. Anal Chem 76(18):5257–5264. https://doi.org/10.1021/ac049837t
Dakhil H, Wierschem A (2014) Measuring low viscosities and high shear rates with a rotational rheometer in a thin-gap parallel-disk configuration. Appl Rheol 24(6):63795–63801
Ewoldt RH, Johnston MT, Caretta LM (2015) Experimental challenges of shear rheology: how to avoid bad data. In: Spagnolie SE (ed) Complex fluids in biological systems: experiment, theory, and computation. Springer, New York, pp 207–241
Dakhil H, Gilbert DF, Malhotra D, Limmer A, Engelhardt H, Amtmann A, Hansmann J, Hübner H, Buchholz R, Friedrich O, Wierschem A (2016) Measuring average rheological quantities of cell monolayers in the linear viscoelastic regime. Rheol Acta 55(7):527–536. https://doi.org/10.1007/s00397-016-0936-5
Furukawa KS, Ushida T, Nagase T, Nakamigawa H, Noguchi T, Tamaki T, Tanaka J, Tateishi T (2001) Quantitative analysis of cell detachment by shear stress. Mater Sci Eng: C 17(1–2):55–58. https://doi.org/10.1016/S0928-4931(01)00336-8
Titze IR, Klemuk SA, Lu X (2012) Adhesion of a monolayer of fibroblast cells to fibronectin under sonic vibrations in a bioreactor. Ann Otol Rhinol Laryngol 121(6):364–374
Pipe CJ, Majmudar TS, McKinley GH (2008) High shear rate viscometry. Rheol Acta 47(5–6):621–642
Dakhil H, Gilbert DF, Malhotra D, Limmer A, Engelhardt H, Amtmann A, Hansmann J, Hübner H, Buchholz R, Friedrich O, Wierschem A (2016) Measuring average rheological quantities of cell monolayers in the linear viscoelastic regime. Rheol Acta:1–10. https://doi.org/10.1007/s00397-016-0936-5
Kokkinos D, Dakhil H, Wierschem A, Briesen H, Braun A (2016) Deformation and rupture of Dunaliella salina at high shear rates without the use of thickeners. Biorheology 53(1):1–11. https://doi.org/10.3233/BIR-15057
Todaro GJ, Green H (1963) Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol 17(2):299–313
Freshney R (1993) Culture of animal cells. A manual of basic techniques, 3rd edn. Wiley-Liss, New York
Goldstein AS, DiMilla PA (2003) Examination of membrane rupture as a mechanism for mammalian cell detachment from fibronectin-coated biomaterials. J Biomed Mater Res Part A 67A(2):658–666. https://doi.org/10.1002/jbm.a.10125
Agudo JR, Dasilva S, Wierschem A (2014) How do neighbors affect incipient particle motion in laminar shear flow?. Phys Fluids 26(5):053303. https://doi.org/10.1063/1.4874604
Agudo JR, Wierschem A (2012) Incipient motion of a single particle on regular substrates in laminar shear flow. Phys Fluids 24(9):093302. https://doi.org/10.1063/1.4753941
Engler AJ, Chan M, Boettiger D, Schwarzbauer JE (2009) A novel mode of cell detachment from fibrillar fibronectin matrix under shear. J Cell Sci 122(10):1647–1653. https://doi.org/10.1242/jcs.040824
Peel MM, DiMilla PA (1999) Effect of cell–cell interactions on the observable strength of adhesion of sheets of cells. Ann Biomed Eng 27(2):236–246. https://doi.org/10.1114/1.178
Akiyama SK, Yamada KM (1985) The interaction of plasma fibronectin with fibroblastic cells in suspension. J Biol Chem 260(7):4492–4500
Culp LA, Sukenik CN (1994) Glass and metal surfaces derivatized with self-assembled monolayers: Cell type-specific modulation of fibronectin adhesion functions. J Tissue Culture Methods 16(3):161–172. https://doi.org/10.1007/BF01540644
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dakhil, H., Do, H., Hübner, H. et al. Measuring the adhesion limit of fibronectin for fibroblasts with a narrow-gap rotational rheometer. Bioprocess Biosyst Eng 41, 353–358 (2018). https://doi.org/10.1007/s00449-017-1868-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-017-1868-x