Abstract
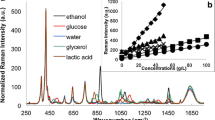
The monitoring of microbiological processes using Raman spectroscopy has gained in importance over the past few years. Commercial Raman spectroscopic equipment consists of a laser, spectrometer, and fiberoptic immersion probe in direct contact with the fermentation medium. To avoid possible sterilization problems and biofilm formation on the probe tip, a large-aperture Raman probe was developed. The design of the probe enables non-contact in-line measurements through glass vessels or inspection glasses of bioreactors and chemical reactors. The practical applicability of the probe was tested during yeast fermentations by monitoring the consumption of substrate glucose and the formation of ethanol as the product. Multiple linear regression models were applied to evaluate the Raman spectra. Reference values were determined by high-performance liquid chromatography. The relative errors of prediction for glucose and ethanol were 5 and 3%, respectively. The presented Raman probe allows simple adaption to a wide range of processes in the chemical, pharmaceutical, and biotechnological industries.





Similar content being viewed by others
References
Geörg D, Schalk R, Methner F-J, Beuermann T (2015) MIR-ATR sensor for process monitoring. Meas Sci Technol 26:065501. doi:10.1088/0957-0233/26/6/065501
Kessler RW (2013) Perspectives in process analysis: process analysis and technology. J Chemom 27:369–378. doi:10.1002/cem.2549
Braun F, Schwolow S, Seltenreich J et al (2016) Highly sensitive Raman spectroscopy with low laser power for fast in-line reaction and multiphase flow monitoring. Anal Chem 88:9368–9374. doi:10.1021/acs.analchem.6b01509
Brun N, Youssef I, Chevrel M-C et al (2013) In situ monitoring of styrene polymerization using Raman spectroscopy. Multi-scale approach of homogeneous and heterogeneous polymerization processes: in situ monitoring of styrene polymerization using Raman spectroscopy. J Raman Spectrosc 44:909–915. doi:10.1002/jrs.4279
De Beer TRM, Allesø M, Goethals F et al (2007) Implementation of a process analytical technology system in a freeze-drying process using Raman spectroscopy for in-line process monitoring. Anal Chem 79:7992–8003. doi:10.1021/ac070549h
Romero-Torres S, Pérez-Ramos JD, Morris KR, Grant ER (2006) Raman spectroscopy for tablet coating thickness quantification and coating characterization in the presence of strong fluorescent interference. J Pharm Biomed Anal 41:811–819. doi:10.1016/j.jpba.2006.01.033
Saerens L, Vervaet C, Remon J-P, De Beer T (2013) Visualization and process understanding of material behavior in the extrusion barrel during a hot-melt extrusion process using raman spectroscopy. Anal Chem 85:5420–5429. doi:10.1021/ac400097t
Vankeirsbilck T, Vercauteren A, Baeyens W et al (2002) Applications of Raman spectroscopy in pharmaceutical analysis. TrAC Trends Anal Chem 21:869–877. doi:10.1016/S0165-9936(02)01208-6
De Beer TRM, Bodson C, Dejaegher B et al (2008) Raman spectroscopy as a process analytical technology (PAT) tool for the in-line monitoring and understanding of a powder blending process. J Pharm Biomed Anal 48:772–779. doi:10.1016/j.jpba.2008.07.023
Chevrel M-C, Hoppe S, Meimaroglou D et al (2016) Application of Raman spectroscopy to characterization of residence time distribution and online monitoring of a pilot-scale tubular reactor for acrylic acid solution polymerization. Macromol React Eng 10:406–414. doi:10.1002/mren.201500055
Cornel J, Mazzotti M (2008) Calibration-Free quantitative application of in situ raman spectroscopy to a crystallization process. Anal Chem 80:9240–9249. doi:10.1021/ac801606z
Šašić S, Ozaki Y (2010) Raman, infrared, and near-infrared chemical imaging. Wiley, Hoboken
Salzer R, Siesler HW (2014) Infrared and Raman spectroscopic imaging. Wiley-VCH, Weinheim (2., completely rev. and updated ed.)
Schwolow S, Braun F, Rädle M et al (2015) Fast and efficient acquisition of kinetic data in microreactors using in-line Raman analysis. Org Process Res Dev 19:1286–1292. doi:10.1021/acs.oprd.5b00184
Shope TB, Vickers TJ, Mann CK (1987) The direct analysis of fermentation products by raman spectroscopy. Appl Spectrosc 41:908–912
Shaw AD, Kaderbhai N, Jones A et al (1999) Noninvasive, on-line monitoring of the biotransformation by yeast of glucose to ethanol using dispersive raman spectroscopy and chemometrics. Appl Spectrosc 53:1419–1428. doi:10.1366/0003702991945777
Sivakesava S, Irudayaraj J, Demirci A (2001) Monitoring a bioprocess for ethanol production using FT-MIR and FT-Raman spectroscopy. J Ind Microbiol Biotechnol 26:185–190
Aarnoutse PJ, Westerhuis JA (2005) Quantitative raman reaction monitoring using the solvent as internal standard. Anal Chem 77:1228–1236. doi:10.1021/ac0401523
Picard A, Daniel I, Montagnac G, Oger P (2007) In situ monitoring by quantitative Raman spectroscopy of alcoholic fermentation by Saccharomyces cerevisiae under high pressure. Extremophiles 11:445–452. doi:10.1007/s00792-006-0054-x
Iversen JA, Berg RW, Ahring BK (2014) Quantitative monitoring of yeast fermentation using Raman spectroscopy. Anal Bioanal Chem 406:4911–4919. doi:10.1007/s00216-014-7897-2
Berger AJ, Itzkan I, Feld MS (1997) Feasibility of measuring blood glucose concentration by near-infrared Raman spectroscopy. Spectrochim Acta Part A 53:287–292
Enejder AMK, Scecina TG, Oh J et al (2005) Raman spectroscopy for noninvasive glucose measurements. J Biomed Opt 10:031114. doi:10.1117/1.1920212
Bechtel KL, Shih W-C, Feld MS (2008) Intrinsic Raman spectroscopy for quantitative biological spectroscopy part II: experimental applications. Opt Express 16:12737–12745
Braun F, Schalk R, Brunner J et al (2016) Nicht-invasive Prozesssonde zur Inline-Ramananalyse durch optische Schaugläser. Tm Tech Mess. doi:10.1515/teme-2016-0011
Schalk R, Geoerg D, Staubach J et al (2016) Evaluation of a newly developed mid-infrared sensor for real-time monitoring of yeast fermentations. J Biosci Bioeng. doi:10.1016/j.jbiosc.2016.12.005
Beuermann T, Egly D, Geoerg D et al (2012) On-line carbon balance of yeast fermentations using miniaturized optical sensors. J Biosci Bioeng 113:399–405. doi:10.1016/j.jbiosc.2011.10.016
Carey WP, Beebe KR, Sanchez E et al (1986) Chemometric analysis of multisensor arrays. Sens Actuators 9:223–234. doi:10.1016/0250-6874(86)80023-3
Preacher KJ, Curran PJ, Bauer DJ (2006) Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat 31:437–448. doi:10.3102/10769986031004437
Bellon V, Vigneau JL, Sévila F (1994) Infrared and near-infrared technology for the food industry and agricultural uses: on-line applications. Food Control 5:21–27. doi:10.1016/0956-7135(94)90129-5
Via B, Shupe T, Groom L et al (2003) Multivariate modelling of density, strength and stiffness from near infrared spectra for mature, juvenile and pith wood of longleaf pine (Pinus palustris). J Infrared Spectrosc 11:365. doi:10.1255/jnirs.388
Livingstone D (2009) A practical guide to scientific data analysis. Wiley, Chichester
Hidalgo B, Goodman M (2013) Multivariate or multivariable regression? Am J Public Health 103:39–40. doi:10.2105/AJPH.2012.300897
Hejazi L, Mohammadi DE, Yamini Y, Brereton RG (2004) Solid-phase extraction and simultaneous spectrophotometric determination of trace amounts of Co, Ni and Cu using partial least squares regression. Talanta 62:183–189. doi:10.1016/S0039-9140(03)00412-0
Rohleder D, Kiefer W, Petrich W (2004) Quantitative analysis of serum and serum ultrafiltrate by means of Raman spectroscopy. Analyst 129:906. doi:10.1039/b408927h
Sivakesava S, Irudayaraj J, Ali D (2001) Simultaneous determination of multiple components in lactic acid fermentation using FT-MIR, NIR, and FT-Raman spectroscopic techniques. Process Biochem 37:371–378. doi:10.1016/S0032-9592(01)00223-0
Söderholm S, Roos YH, Meinander N, Hotokka M (1999) Raman spectra of fructose and glucose in the amorphous and crystalline states. J Raman Spectrosc 30:1009–1018
Vasko PD, Blackwell J, Koenig JL (1972) Infrared and raman spectroscopy of carbohydrates.: part II: normal coordinate analysis of α-d-glucose. Carbohydr Res 23:407–416
Mazarevica G, Diewok J, Baena JR et al (2004) On-line fermentation monitoring by mid-infrared spectroscopy. Appl Spectrosc 58:804–810. doi:10.1366/0003702041389229
Ibrahim M, Alaam M, El-Haes H et al (2006) Analysis of the structure and vibrational spectra of glucose and fructose. Eclética Quím 31:15–21. doi:10.1590/S0100-46702006000300002
Günzler H (2002) IR spectroscopy: an introduction. Wiley-VCH, Weinheim
Gan W, Zhang Z, Feng R, Wang H (2006) Identification of overlapping features in the sum frequency generation vibrational spectra of air/ethanol interface. Chem Phys Lett 423:261–265. doi:10.1016/j.cplett.2006.03.084
Ávila TC, Poppi RJ, Lunardi I et al (2012) Raman spectroscopy and chemometrics for on-line control of glucose fermentation by Saccharomyces cerevisiae. Biotechnol Prog 28:1598–1604. doi:10.1002/btpr.1615
Wang Q, Li Z, Ma Z, Liang L (2014) Real time monitoring of multiple components in wine fermentation using an on-line auto-calibration Raman spectroscopy. Sens Actuators B Chem 202:426–432. doi:10.1016/j.snb.2014.05.109
Berry B, Moretto J, Matthews T et al (2015) Cross-scale predictive modeling of CHO cell culture growth and metabolites using Raman spectroscopy and multivariate analysis. Biotechnol Prog 31:566–577. doi:10.1002/btpr.2035
André S, Cristau LS, Gaillard S et al (2015) In-line and real-time prediction of recombinant antibody titer by in situ Raman spectroscopy. Anal Chim Acta 892:148–152. doi:10.1016/j.aca.2015.08.050
Acknowledgements
We gratefully acknowledge the support from the Albert and Anneliese Konanz-Foundation of the Mannheim University of Applied Sciences. We would also like to thank the Institute for Technical Microbiology (Mannheim University of Applied Sciences, Germany), especially Kerstin Schlosser, for providing the HPLC system. Furthermore, the authors would like to thank Dr. Hanns Simon Eckhardt (tec5 AG, Germany) for technical support. This work was funded by the German Federation of Industrial Research Associations (AiF Project GmbH, Funding Code 2035756LW3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Rights and permissions
About this article
Cite this article
Schalk, R., Braun, F., Frank, R. et al. Non-contact Raman spectroscopy for in-line monitoring of glucose and ethanol during yeast fermentations. Bioprocess Biosyst Eng 40, 1519–1527 (2017). https://doi.org/10.1007/s00449-017-1808-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-017-1808-9