Abstract
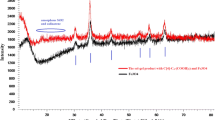
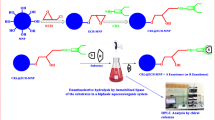
This work presents the synthesis of new mercapto calix[4]arenes derivatives (4 and 5). These derivatives were capped on Fe3O4 magnetic nanoparticles and subsequently encapsulated with Candida rugosa through sol–gel method to furnish enc-4 and enc-5, respectively, to enhance catalytic activity and enantioselectivity of lipase for hydrolysis reaction of racemic flurbiprofen methyl ester. Catalytic activity and enantioselectivity of enc-4 and enc-5 were assayed at different pH and temperature conditions and it was found that the resultant encapsulated enzyme exhibited higher thermal and operational stabilities compared to the free lipase in which enc-5 showed the excellent rate of enantioselectivity (E = 176) for S-flurbiprofen better than free lipase (E = 137) at pH 7 and 35 °C for 48 h. The time study shows that enantioselectivity reached the maximum value of E = 244 after 72 h. Catalytic activity of these materials was hardly affected by 20 and 23% after five usages of enc-4 and enc-5, respectively.








Similar content being viewed by others
References
Grösch S, Schilling K, Janssen A, Maier TJ, Niederberger E, Geisslinger G (2005) Induction of apoptosis by R-flurbiprofen in human colon carcinoma cells: involvement of p53. Biochem Pharmacol 69(5):831–839
Murali Mohan Babu G, Prasad CD, Himasankar K, Gourishankar V, Kumar NK, Ramana Murthy K (2002) Development of new controlled release formulation of flurbiprofen: in vitro-in vivo correlation. Indian J Pharm Sci 64(1):37–43
Muraoka A, Tokumura T, Machida Y (2004) Evaluation of the bioavailability of flurbiprofen and its β-cyclodextrin inclusion complex in four different doses upon oral administration to rats. Eur J Pharm Biopharm 58(3):667–671
Williams R, Jeffcoat M, Kaplan M, Goldhaber P, Johnson H, Wechter W (1985) Flurbiprofen: a potent inhibitor of alveolar bone resorption in beagles. Science 227(4687):640–642
Eriksen JL, Sagi SA, Smith TE, Weggen S, Das P, McLendon D, Ozols VV, Jessing KW, Zavitz KH, Koo EH (2003) NSAIDs and enantiomers of flurbiprofen target γ-secretase and lower Aβ42 in vivo. J Clin Investig 112(3):440–449
Uchino T, Matsumoto Y, Murata A, Oka T, Miyazaki Y, Kagawa Y (2014) Transdermal delivery of flurbiprofen from surfactant-based vesicles: particle characterization and the effect of water on in vitro transport. Int J Pharm 464(1):75–84
Pignatello R, Bucolo C, Spedalieri G, Maltese A, Puglisi G (2002) Flurbiprofen-loaded acrylate polymer nanosuspensions for ophthalmic application. Biomaterials 23(15):3247–3255
Rousseau A, Chiap P, Ivanyi R, Crommen J, Fillet M, Servais A-C (2008) Validation of a nonaqueous capillary electrophoretic method for the enantiomeric purity determination of R-flurbiprofen using a single-isomer amino cyclodextrin derivative. J Chromatogr A 1204(2):219–225
Białońska A, Ciunik Z (2004) Racemic resolution of N-protected alanine by strychnine and brucine versus donor/acceptor capability. CrystEngComm 6(47):276–279
Wulff G, Poll HG (1987) Enzyme-analogue built polymers, 23. Influence of the structure of the binding sites on the selectivity for racemic resolution. Die. Makromol Chem 188(4):741–748
Ranjbakhsh E, Bordbar A, Abbasi M, Khosropour A, Shams E (2012) Enhancement of stability and catalytic activity of immobilized lipase on silica-coated modified magnetite nanoparticles. Chem Eng J 179:272–276
Zhang D-H, Zhang Y-F, Zhi G-Y, Xie Y-L (2011) Effect of hydrophobic/hydrophilic characteristics of magnetic microspheres on the immobilization of BSA. Colloids Surf B 82(2):302–306. doi:10.1016/j.colsurfb.2010.09.001
Andrade LH, Rebelo LP, Netto CGCM, Toma HE (2010) Kinetic resolution of a drug precursor by Burkholderia cepacia lipase immobilized by different methodologies on superparamagnetic nanoparticles. J Mol Catal B Enzym 66(1–2):55–62. doi:10.1016/j.molcatb.2010.03.002
Köse Ö, Tüter M, Aksoy HA (2002) Immobilized Candida antarctica lipase-catalyzed alcoholysis of cotton seed oil in a solvent-free medium. Bioresour Technol 83(2):125–129. doi:10.1016/S0960-8524(01)00203-6
Chang SW, Shaw JF, Yang KH, Chang SF, Shieh CJ (2008) Studies of optimum conditions for covalent immobilization of Candida rugosa lipase on poly(γ-glutamic acid) by RSM. Bioresour Technol 99(8):2800–2805. doi:10.1016/j.biortech.2007.06.020
Dlugy C, Wolfson A (2007) Lipase catalyse glycerolysis for kinetic resolution of racemates. Bioproc Biosyst Eng 30(5):327–330. doi:10.1007/s00449-007-0128-x
Houde A, Kademi A, Leblanc D (2004) Lipases and their industrial applications. Appl Biochem Biotechnol 118(1–3):155–170
Sayin S, Yilmaz E, Yilmaz M (2011) Improvement of catalytic properties of Candida Rugosa lipase by sol–gel encapsulation in the presence of magnetic calix[4]arene nanoparticles. Org Biomol Chem 9(11):4021–4024. doi:10.1039/c1ob05115f
Uyanik A, Sen N, Yilmaz M (2016) Enhancing effect of calix[4]arene amide derivatives on lipase performance in enantioselective hydrolysis of racemic arylpropionic acid methyl esters. Polycycl Aromat Comp 36(5):613–627. doi:10.1080/10406638.2015.1037005
Sayin S, Yilmaz M (2014) Bronsted acidic magnetic nano-Fe3O4-adorned calix[n]arene sulfonic acids: synthesis and application in the nucleophilic substitution of alcohols. Tetrahedron 70(37):6669–6676. doi:10.1016/j.tet.2014.06.034
Akceylan E, Uyanik A, Eymur S, Sahin O, Yilmaz M (2015) Calixarene-proline functionalized iron oxide magnetite nanoparticles (Calix-Pro-MN): an efficient recyclable organocatalyst for asymmetric aldol reaction in water. Appl Catal a-Gen 499:205–212. doi:10.1016/j.apcata.2015.04.018
Reetz MT, Tielmann P, Wiesenhöfer W, Könen W, Zonta A (2003) Second generation sol–gel encapsulated lipases: robust heterogeneous biocatalysts. Adv Synth Catal 345(6–7):717–728
Yilmaz E, Sezgin M, Yilmaz M (2011) Immobilization of Candida rugosa lipase on magnetic sol–gel composite supports for enzymatic resolution of (R, S)-naproxen methyl ester. J Mol Catal B Enzym 69(1):35–41
Sayin S, Akoz E, Yilmaz M (2014) Enhanced catalysis and enantioselective resolution of racemic naproxen methyl ester by lipase encapsulated within iron oxide nanoparticles coated with calix[8]arene valeric acid complexes. Org Biomol Chem 12(34):6634–6642. doi:10.1039/c4ob01048e
Uyanik A, Sen N, Yilmaz M (2011) Improvement of catalytic activity of lipase from Candida rugosa via sol–gel encapsulation in the presence of calix(aza)crown. Bioresource Technol 102(6):4313–4318. doi:10.1016/j.biortech.2010.12.105
Ozyilmaz E, Sayin S (2013) Preparation of new calix [4] arene-immobilized biopolymers for enhancing catalytic properties of Candida rugosa lipase by sol–gel encapsulation. Appl Biochem Biotechnol 170(8):1871–1884
Sahin O, Erdemir S, Uyanik A, Yilmaz M (2009) Enantioselective hydrolysis of (R/S)-naproxen methyl ester with sol–gel encapculated lipase in presence of calix[n]arene derivatives. Appl Catal A 369(1):36–41
Itoh T, Mitsukura K, Kanphai W, Takagi Y, Kihara H, Tsukube H (1997) Thiacrown ether technology in lipase-catalyzed reaction: scope and limitation for preparing optically active 3-hydroxyalkanenitriles and application to insect pheromone synthesis. J Org Chem 62(26):9165–9172
Fadnavis N, Babu RL, Vadivel SK, Deshpande AA, Bhalerao U (1998) Lipase catalyzed regio-and stereospecific hydrolysis: chemoenzymatic synthesis of both (R)- and (S)-enantiomers of α-lipoic acid. Tetrahedron Asymmetry 9(23):4109–4112
Wu J-Y, Liu S-W (2000) Influence of alcohol concentration on lipase-catalyzed enantioselective esterification of racemic naproxen in isooctane: under controlled water activity. Enzyme Microb Technol 26(2):124–130
Collins EM, McKervey MA, Madigan E, Moran MB, Owens M, Ferguson G, Harris SJ (1991) Chemically modified calix [4]arenes. Regioselective synthesis of 1, 3-(distal) derivatives and related compounds. X-Ray crystal structure of a diphenol-dinitrile. J Chem Soc Perkin Trans 1(12):3137–3142
Gutsche CD, Iqbal M, Stewart D (1986) Calixarenes. 19. Syntheses procedures for p-tert-butylcalix [4] arene. J Org Chem 51(5):742–745
Xu W, Li J-S, Feng Y-Q, Da S-L, Chen Y-Y, Xiao X-Z (1998) Preparation and characterization ofp-tert-butyl-calix [6] arene-bonded silica gel stationary phase for high-performance liquid chromatography. Chromatographia 48(3–4):245–250
Maity D, Chakraborty A, Gunupuru R, Paul P (2011) Calix[4]arene based molecular sensors with pyrene as fluoregenic unit: effect of solvent in ion selectivity and colorimetric detection of fluoride. Inorg Chim Acta 372(1):126–135. doi:10.1016/j.ica.2011.01.053
Fu Y-Q, Li Z-C, Ding L-N, Tao J-C, Zhang S-H, Tang M-S (2006) Direct asymmetric aldol reaction catalyzed by simple prolinamide phenols. Tetrahedron Asymmetry 17(24):3351–3357. doi:10.1016/j.tetasy.2006.12.008
Akoz E, Erdemir S, Yilmaz M (2012) Immobilization of novel the semicarbazone derivatives of calix[4]arene onto magnetite nanoparticles for removal of Cr(VI) ion. J Incl Phenom Macro 73(1–4):449–458. doi:10.1007/s10847-011-0083-7
Sayin S, Yilmaz M (2011) Preparation and uranyl ion extraction studies of calix[4]arene-based magnetite nanoparticles. Desalination 276(1–3):328–335. doi:10.1016/j.desal.2011.03.073
Chiou S-H, Wu W-T (2004) Immobilization of Candida rugosa lipase on chitosan with activation of the hydroxyl groups. Biomaterials 25(2):197–204
Bonnet M, Leroux C, Chilliard Y, Martin P (2001) A fluorescent reverse transcription—polymerase chain reaction assay to quantify the lipoprotein lipase messenger RNA. Mol Cell Probes 15(4):187–194
Chen CS, Fujimoto Y, Girdaukas G, Sih CJ (1982) Quantitative analyses of biochemical kinetic resolutions of enantiomers. J Am Chem Soc 104(25):7294–7299
Zhu S, Wu Y, Yu Z (2005) Immobilization of Candida rugosa lipase on a pH-sensitive support for enantioselective hydrolysis of ketoprofen ester. J Biotechnol 116(4):397–401
Aktas M, Uyanik A, Eymur S, Yilmaz M (2016) l-Proline derivatives based on a calix[4]arene scaffold as chiral organocatalysts for the direct asymmetric aldol reaction in water. Supramol Chem 28(5–6):351–359. doi:10.1080/10610278.2015.1073288
Pereira EB, De Castro HF, De Moraes FF, Zanin GM (2001) Kinetic studies of lipase from Candida rugosa. Appl Biochem Biotechnol 91(1–9):739–752
Acknowledgements
We would like to thank The Research Foundation of Selcuk University (BAP) for their financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yildiz, H., Ozyilmaz, E., Bhatti, A.A. et al. Enantioselective resolution of racemic flurbiprofen methyl ester by lipase encapsulated mercapto calix[4]arenes capped Fe3O4 nanoparticles. Bioprocess Biosyst Eng 40, 1189–1196 (2017). https://doi.org/10.1007/s00449-017-1779-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-017-1779-x